Why EBER ISH Isn’t the Same as EBV IHC And Why It Matters

By admin
Introduction: The Molecular Mandate for Accuracy
When a head-and-neck surgeon sends up a nasopharyngeal biopsy, the pathologist has one job: to tell the team whether EBV is hiding inside those cancer cells. Get it wrong and the treatment plan falls apart. For years, labs have used two main tests to look for the virus—EBER ISH and EBV IHC—and too many people treat them like they’re the same thing. They’re not. One is looking for RNA that’s basically indestructible; the other is chasing proteins that can disappear depending on what mood the virus is in that day. Mix them up, and the patient loses.
The Diagnostic Dilemma of Viral Cancers
EBV doesn’t act like most viruses. After the initial infection (usually in childhood), it parks itself inside B-cells or epithelial cells and goes almost completely silent. That silent phase is called latency, and it’s the reason EBV can cause cancer decades later. The virus only turns on a handful of genes, and which genes depend on the type of latency—I, II, or III. The test you pick has to match what the virus is actually making.
Setting the Stage: RNA vs. Protein Detection
EBER ISH goes straight for the RNA. EBV IHC goes after proteins. That single difference decides whether you catch every positive case or let some slip through the cracks.
Deciphering the Molecular Targets
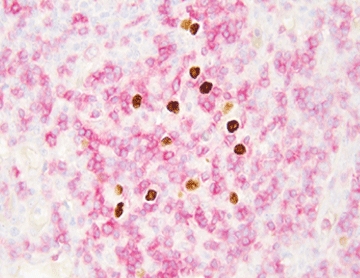
EBER ISH: Targeting the Abundant Viral RNA (The Gold Standard)
EBER stands for Epstein-Barr Encoded RNA—two tiny non-coding RNAs called EBER1 and EBER2. They sit in the nucleus doing whatever they do, but the important part is how many there are: up to ten million copies per infected cell. Ten million. And because they fold into tight little balls, they laugh at the chemicals we use to fix tissue. Even in a block that’s been sitting on a shelf for fifteen years, EBER is usually still there, ready to light up. The probe sticks to those millions of copies, and the nucleus turns black. Clean, sharp, impossible to argue with. Celnovte built Super ISH™ to make that signal even stronger and the background snow-white, no matter how beat-up the tissue is.
EBV IHC: Targeting Variable Latent Proteins (The Pitfalls of Selectivity)
IHC looks for proteins like LMP1, EBNA2, or sometimes EBNA1. Sounds good, except the virus only makes those proteins when it feels like it. In nasopharyngeal carcinoma, you usually get Latency II—LMP1 is there, but often weak, and EBNA2 is completely off. In Burkitt lymphoma, Latency I—LMP1 and EBNA2 are gone, only EBNA1 hangs around. If your antibody is against LMP1 and the tumor is Latency I, you get a big fat negative even though the virus is very much present. Add in over-fixation or under-fixation, and the protein folds the wrong way or washes out completely. Suddenly, the stain is blank and the pathologist is left guessing.
Clinical Ramifications: The Imperative for EBER ISH
Diagnostic Sensitivity and Consensus in Lymphoproliferative Disorders
Every major guideline says the same thing: for nasopharyngeal carcinoma, the tumor is EBV-related only if EBER is positive. Period. LMP1 can be nice to see, but it’s not required because it misses too many cases. Same story in lymphomas—big diffuse large B-cell tumors in older patients can be EBV-driven even when LMP1 is barely there. Miss it with IHC, and the prognosis call changes completely.
The Challenge of Latency Type and Protein Heterogeneity
Even inside the same tumor, some areas can run one latency program and other areas another. Protein staining ends up patchy; one field looks positive, the next looks negative. EBER stays rock-steady—every tumor cell with virus shows that strong nuclear dot. No debate, no “weak positive” calls that make tumor boards argue for twenty minutes.
Research Spotlight: Quantifying the Difference
Look at any decent study comparing the two methods, and the numbers tell the story. In nasopharyngeal carcinoma series, EBER ISH is positive in 95–100 % of cases. LMP1 IHC? Often only 60–80 %. That gap is real patients who would have been called EBV-negative if the lab had only run IHC.
Celnovte’s Integrated Precision Pathology Solutions
Super ISH™ Technology: Achieving Single-Cell Resolution
Celnovte launched Super ISH™ in 2020 because pathologists kept complaining that regular ISH kits gave a dirty background on gastric and tonsil cases. Super ISH™ fixed it. The signal is bright, the dots are crisp, and even low-copy RNA targets pop out clearly. For EBER, it’s overkill in the best way—every positive nucleus looks textbook perfect.
MicroStacker™ Detection Systems: Ensuring Unsurpassed Sensitivity for Companion Markers
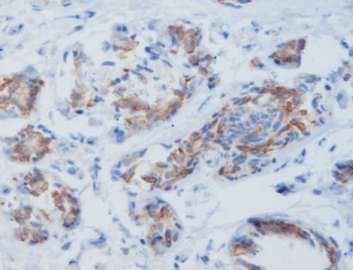
Sometimes you still need to see LMP1 or other proteins. MicroStacker™ polymer gives a cleaner, stronger brown than most kits on the market. The newest version, MicroStacker™ Ultra, pushes the sensitivity even higher so weak expressors don’t get missed. Labs run it for everything from PD-L1 to MMR proteins and get the same reliable staining day after day.
CNT 360 Stainer: Automation for Integrated Workflows
The CNT 360 is the bench-top workhorse that does both IHC and ISH without breaking a sweat. Load the slides in the morning, pick Super ISH™ for the EBER run and MicroStacker™ for the LMP1 controls, and come back to finished racks. Since 2018, Celnovte has put more than 800 automated stainers into labs worldwide, and the feedback is always the same: turnaround time drops, repeats disappear, and the staining looks identical whether the tech started the run at 7 a.m. or 7 p.m.

Conclusion: Elevating Diagnostics for Patient Care
If the question is “Is EBV in this tumor?”, the answer has to be EBER ISH. It catches every latency type, survives terrible fixation, and gives a yes-or-no signal nobody can misread. Run LMP1 IHC if you want to know exactly which proteins are on, but never use it to rule the virus out.
Celnovte built an entire system around getting this right—Super ISH™ for bulletproof RNA detection, MicroStacker™ when you need protein details, and the CNT 360 to make the whole process fast and repeatable. When an oncologist is deciding radiation fields or whether to add immunotherapy, they deserve the most reliable answer possible. That answer starts with the right test.
FAQ
Q: Why is EBER ISH considered the gold standard for latent EBV detection?
A: EBER RNA is produced in massive amounts and stays stable no matter how the tissue was fixed, so the test almost never misses a positive case.
Q: Which Celnovte technology is designed to optimize EBER detection?
A: Super ISH™ RNA In-Situ Hybridization Technology gives single-molecule, single-cell clarity and the cleanest background available.
Q: How many fully automated IHC stainers has Celnovte installed globally?
A: Over 800 units since 2018, running in hospitals and reference labs on every continent.
Q: What is the primary benefit of using the CNT 360 Stainer?
A: It runs both IHC and ISH protocols side by side with fast turnaround and perfect consistency, no matter how many slides you load.
RELATED PRODUCTS






