-
Home
-
Product

-
IMMUNOHISTOCHEMISTRY

-
INSTRUMENT

-
MOLECULAR

-
HISTOLOGY

-
CYTOLOGY

-
DIGITAL PATHOLOGY

-
-
Solution

-
Support

-
About

-
Contact



About Celnovte
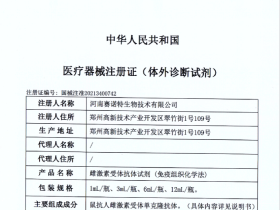
Celnovte Biotechnology Co., Ltd (Celnovte) is specialized in R&D, manufacturing and distribution of pathological diagnostic reagents and instruments. Celnovte has a market-leading portfolio of Immunohistochemistry and in-situ hybridization. Celnovte's MicroStacker™ IHC detection system is renowned for its unsurpassed sensitivity and specificity. Celnovte's PolyStacker™ technology drastically reduces the turnaround time of frozen section IHC experiment to as short as 10mins. Celnovte's SuperISH™ RNA in-situ hybridization technology enables detection of RNA targets at the single molecular level and single cell resolution. Celnovte is also leading in its innovation of fully automated instrumentation, such as IHC stainers, H&E stainer, special stainer, cytopathology instrumentation and digital slide scanner and etc. Since their launch in 2018, Celnovte have successfully installed over 600 units of fully automated IHC stainers globally.
Founded in 2010, Celnovte is headquartered in Zhengzhou, China for reagent and instrument development and manufacturing. It also has subsidiaries in Shenzhen for instrument R&D, in Suzhou for research-use product development, and in Maryland(USA) for reagent R&D. Our facilities in China are NMPA & GMP compliant and are certified for ISO13485 and ISO9001. Nowadays, Celnovte has established an extensive domestic and international pathological network, reaching over 1500+ top hospitals in China and over 10+ countries worldwide.
Our mission is to elevate precision in cancer diagnostics and enrich patients' lives through innovative products and services. We aim to be a global leader in delivering top-quality diagnostic services by prioritizing product quality, unmatched customer satisfaction, advancing scientific innovation, and building a premier organization committed to make better, do better, and be better for our customers, employees, partners, and the patient worldwide.
Story
- Established Celnovte Biotechnology Co.,Ltd
2010
2010
- Established Celnovte Biotechnology Co.,Ltd
- Obtained IVD reagent production licence
- Launched mycobacterium tuberculosis culture and drug susceptibility identification product lines
2011
2011
- Obtained IVD reagent production licence
- Launched mycobacterium tuberculosis culture and drug susceptibility identification product lines
- Launched immunohistochemical products R&D platform
2012
2012
- Launched immunohistochemical products R&D platform
- Launched FISH R&D platform
- Established pathology diagnosis technology engineering research center
2013
2013
- Launched FISH R&D platform
- Established pathology diagnosis technology engineering research center
- Established histopathological staining R&D platform
2014
2014
- Established histopathological staining R&D platform
- Established 10,000 and 100,000 class cleaning-room 2000 square meters
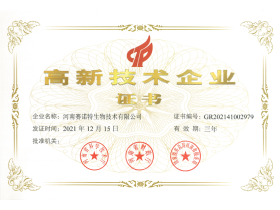
- Won national high-tech enterprise title
- Established Henan Province tumor pathology diagnostic reagent engineering research center
2015
2015
- Established 10,000 and 100,000 class cleaning-room 2000 square meters
- Won national high-tech enterprise title
- Established Henan Province tumor pathology diagnostic reagent engineering research center
- Launched 429 immunohistochemical primary antibody
2016
2016
- Launched 429 immunohistochemical primary antibody
- Launched over 100 FISH probes
- Established Zhengzhou tumor pathological accurate diagnosis academician workstation
2017
2017
- Launched over 100 FISH probes
- Established Zhengzhou tumor pathological accurate diagnosis academician workstation
- Established a branch office in the U.S.
- Established a branch office in Shenzhen
- Established Celnovte ICL
- Established pathological diagnosis postdoctoral program
2018
2018
- Established a branch office in the U.S.
- Established a branch office in Shenzhen
- Established Celnovte ICL
- Established pathological diagnosis postdoctoral program
- Met ISO13485 and ISO9001 standard
- Launched MicroStacker™ Polymer with our own autonomous kernel technology
- Launched IHC Stainer CNT 300 / 330
- Established a new production base of 5000 square meters
2019
2019
- Met ISO13485 and ISO9001 standard
- Launched MicroStacker™ Polymer with our own autonomous kernel technology
- Launched IHC Stainer CNT 300 / 330
- Established a new production base of 5000 square meters
- Launched the international market
- CE Mark approved
- Launched DualStacker™ Multiplex solutions
- Launched PolyStacker™ Frozen IHC solutions
2020
2020
- Launched the international market
- CE Mark approved
- Launched DualStacker™ Multiplex solutions
- Launched PolyStacker™ Frozen IHC solutions
- Launched Super ISH™ products
- Launched Fully Automatic IHC Stainer with High Throughput CNT 360
- Launched Digital Slide Scanner CNT 160
2021
2021
- Launched Super ISH™ products
- Launched Fully Automatic IHC Stainer with High Throughput CNT 360
- Launched Digital Slide Scanner CNT 160
- Launched Fully Automatic Special Staining, Drop-staining and Coverslipper System CNT520
- Launched Fully Automatic Liquid-based Cytology Immunocytochemistry Stainer CNT480
- IHC stainer marked 600+ installations
2023
2023
- Launched Fully Automatic Special Staining, Drop-staining and Coverslipper System CNT520
- Launched Fully Automatic Liquid-based Cytology Immunocytochemistry Stainer CNT480
- IHC stainer marked 600+ installations
- Launched MicroStacker™ Ultra, the state-of-art technology in the market
2024
2024
- Launched MicroStacker™ Ultra, the state-of-art technology in the market