Validated IHC Biomarkers for ADC Therapy – Celnovte ADC Companion Diagnostic Antibodies
2025-06-11
By celnovte
Introducing Celnovte’s ADC Companion Diagnostic Antibody Series
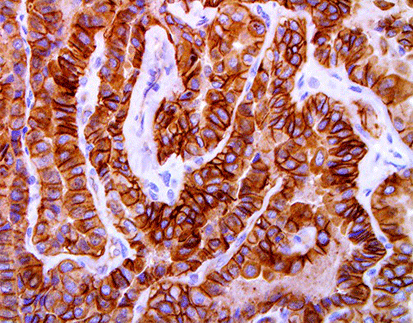
As antibody-drug conjugates (ADCs) gain traction in modern oncology, the need for reliable IHC markers for ADC therapy has become a critical factor in enabling precision treatment. Accurate detection of ADC targets in tumor tissue is the first and most vital step in determining therapeutic eligibility.
Celnovte—a trusted leader in immunohistochemistry—introduces its ADC Companion Diagnostic Antibody Series, developed for clinical use in ADC biomarker screening, pathology laboratories, and drug development programs.
Why Choose Celnovte ADC IHC Markers?
Unlike standard IVD antibodies, Celnovte’s ADC diagnostic antibodies are:
– Clinically validated across real-world FFPE tissue samples
– Used in patient enrollment for ADC clinical trials
– Co-developed with biopharma partners for CDx development
– Optimized for high-specificity, low-background IHC staining
Whether you’re a hospital, CRO, or drug developer, these antibodies are engineered to meet the real-world demands of ADC biomarker detection.
ADC IHC Biomarker Targets Aligned with Clinical Needs
Celnovte’s IHC markers include both FDA-approved and investigational ADC targets, supporting a robust diagnostic portfolio across multiple cancer types:
| ADC Target | Cancer Types | Related ADC Drugs |
|---|---|---|
| HER2 | Breast, gastric, lung | Enhertu, Kadcyla |
| TROP2 | TNBC, NSCLC | Trodelvy |
| Claudin18.2 | Gastric, pancreatic | Zolbetuximab |
| Nectin-4 | Urothelial, breast | Padcev |
| HER3 | NSCLC | Patritumab Deruxtecan |
| B7-H3 | Neuroblastoma, lung | MGC018, DS-7300 (investigational) |
| B7-H4 | Ovarian, breast | ABBV-428, SGN-B7H4V (investigational) |
| FRα (Folate Receptor Alpha) | Ovarian, NSCLC | Mirvetuximab soravtansine |
| c-Met | Liver, gastric, lung | Telisotuzumab vedotin (investigational) |
These IHC biomarkers for ADCs are rigorously optimized for automated staining platforms and clinically validated.
Fully Integrated Platform for ADC Biomarker Testing
To streamline clinical workflows, Celnovte offers an end-to-end solution for ADC IHC diagnostics:
– CNT-Series Automated IHC Stainers — for standardized, high-throughput processing
– Validated protocols and scoring systems to ensure consistency
– High-contrast, low-background staining with advanced detection kits
– Positive/negative controls and tissue reference panels for quality assurance
This fully integrated platform accelerates the companion diagnostic workflow for ADC therapy—from biopsy to clinical decision.
Applications Across the ADC Development Pipeline
Celnovte’s IHC antibodies are actively used in:
– Hospital pathology labs for ADC companion diagnostics
– Biopharma trials to screen patient eligibility
– CROs for assay development and validation
– Translational research in biomarker discovery and distribution studies
With growing demand for CDx IHC markers for ADCs, Celnovte provides clinical-grade reagents backed by scientific collaboration and international validation.
Conclusion: Elevating ADC Precision with IHC Biomarkers That Deliver
Every successful ADC therapy begins with the right IHC biomarker. At Celnovte, we deliver more than antibodies—we deliver validated, ready-to-use diagnostic tools that drive precision oncology.
Whether you’re searching for IHC markers for ADCs, building a companion diagnostic assay, or targeting new tumor types—our portfolio is built for speed, accuracy, and clinical reliability.
📩 Contact us today for technical data sheets, validation reports, or to request a trial sample set of our ADC IHC markers.