The CISH Process: From Probe Hybridization to Signal Detection

By admin

In the detailed world of precision oncology, seeing genetic changes right inside the tissue structure proves essential. For many years, Fluorescence In-Situ Hybridization (FISH) served as the main way to carry out this kind of testing. Now, Chromogenic In-Situ Hybridization (CISH) has changed things by letting pathologists examine molecular targets with a regular light microscope. As a leading supplier of pathological diagnostic tools, Celnovte stands at the forefront of this shift. The company provides modern options like the Super-ISH™ technology to make molecular findings both easy to access and highly accurate.
The Evolution of Molecular Pathology: Why CISH Matters
The chief aim of molecular pathology lies in spotting particular DNA or RNA sequences that play a role in disease. CISH does this through marked probes that attach to matching genetic parts in tissue samples. A color-based reaction then creates a lasting, visible mark.
Bridging the Gap between Morphology and Genetics
A major hurdle in molecular tests comes from losing sight of the tissue layout. CISH makes it possible to check gene status and cell shape at the same time. The signals develop with common staining methods, such as DAB, so the tissue stays clear. This lets the pathologist verify that the genetic mark comes from cancer cells alone, not from nearby supportive tissue.
CISH vs. FISH: The Practical Advantages of Light Microscopy
FISH calls for costly fluorescence scopes and deals with signals that weaken over time. CISH, on the other hand, gives permanent results. Slides can be kept for years and reviewed without trouble in team discussions. For a high-tech company like Celnovte, which started in 2010 with a focus on raising diagnostic accuracy, offering tools that fit smoothly into current lab routines ranks high on the list.
The Step-by-Step CISH Workflow: From Tissue to Diagnosis
The CISH method follows a careful protocol that can take hours or days. It demands tight control over conditions to protect the nucleic acids and keep the probe binding exact.
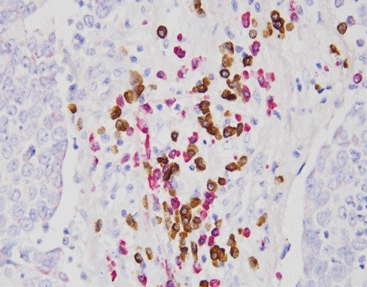
Pre-treatment and Protease Digestion: Preparing the Target
Everything starts with reading the Formalin-Fixed Paraffin-Embedded (FFPE) tissue. To let the genetic probes get to their targets, the sample goes through heat treatment and enzyme breakdown. These steps undo the protein links made during fixation. As a result, the DNA or RNA becomes open for the next phase.
Probe Denaturation and Hybridization: The Core Interaction
With the target ready, a marked probe—built to fit the chosen sequence—gets added. Heat first separates both the probe and the target DNA into single strands. Then, as the temperature drops during hybridization, the probe binds firmly to its matching spot. Celnovte’s strong research setup ensures these probes deliver top binding strength while keeping background stains low.
Post-hybridization Stringency Washes: Ensuring Specificity
Once hybridization finishes, strict washing steps clear away any probes that attached weakly or in the wrong places. This part matters a great deal. Washes that are too gentle leave extra staining; ones that are too strong can wash away real signals. Many labs now turn to automated systems like the CNT 360 for steady control here.
Signal Visualization: The HRP-DAB Chromogenic System
The last step brings the signal into view. Most CISH setups, including Celnovte’s, use a Horseradish Peroxidase (HRP) enzyme approach. The enzyme links to the marked probe and turns a substrate like DAB into a brown deposit that stays put. Under a 40x or 100x lens, gene copies or RNA pieces show up as clear dots or groups.
Celnovte’s Super-ISH™ Technology: Redefining Sensitivity
To overcome the limits of standard CISH, Celnovte created the Super-ISH™ system. This platform marks a big step up in detection power and clarity, opening the door to broader clinical uses.
Single-Molecular Detection and Single-Cell Resolution
Super-ISH™ can spot RNA targets down to the individual molecule level. A strong amplification method makes even scarce transcripts visible with detail at the single-cell scale. This ability helps greatly when looking for viral presence or uncommon gene activity that older methods might overlook.
Featured Products: Kappa/Lambda and EBER Probe Kits
Celnovte supplies a wide range of CISH items, among them the Kappa/Lambda Probe Kit and the EBER Probe. The EBER (Epstein-Barr Virus-Encoded RNA) in-situ hybridization has earned its place as the worldwide benchmark for spotting EBV infection in tissue samples. By offering both manual and automated forms of these probes, Celnovte gives labs of every size the choices they need.
Automation and Precision: Streamlining the CISH Workflow
With rising numbers of samples, automation has become a must rather than a nice extra. Celnovte meets this demand through combined equipment and software packages.
The CNT 360 Advantage: High-Throughput Consistency
The CNT 360 Full Automatic IHC & ISH Stainer works as a complete, powerful machine built to run various staining and in-situ hybridization tasks on one unit. It handles up to 60 slides and finishes in about 2.5 hours. This speed suits the busy needs of today’s diagnostic labs. Automation cuts down on mistakes that can happen during tricky washing or timing steps, bringing unmatched, steady results.

Quality Assurance: NMPA, GMP, and International Standards
Trust in CISH rests on solid quality checks. Celnovte’s production sites in China and the USA meet NMPA & GMP rules and hold ISO13485 and ISO9001 approvals. More than 1,000 automated stainers now run worldwide, and products reach over 40 countries. Real clinical use in more than 2,300 leading hospitals confirms the strength of Celnovte’s CISH tools.
In conclusion, the CISH process builds an important link between classic tissue study and current molecular genetics. By joining the clear tissue views of light microscopy with the sharp detection of Super-ISH™ technology and the fast pace of the CNT 360 stainer, Celnovte helps pathologists give more exact cancer readings. In the end, this leads to better patient care across the globe.
FAQ
Q: What is the primary difference between CISH and FISH?
A: CISH uses a chromogenic reaction observable under a standard light microscope, whereas FISH requires a fluorescence microscope and has signals that fade over time.
Q: Can Celnovte’s Super-ISH™ detect low-abundance RNA?
A: Yes, Super-ISH™ is designed for single-molecular detection, allowing for the visualization of RNA targets at single-cell resolution with high sensitivity.
Q: Is the EBER probe considered a diagnostic standard?
A: Yes, EBER in situ hybridization is widely recognized as the gold standard for the diagnosis of EBV infection in tissue pathology.
Q: How many slides can the CNT 360 process at once?
A: The CNT 360 has a throughput of 60 slides and can complete the staining process in as little as 2.5 hours.
RELATED PRODUCTS






