P16/Ki-67 Dual Staining in Cervical Cancer Screening
 2024-02-01
2024-02-01
By celnovte
Summary
Aiding Cervical Cancer Screening with a Comprehensive Solution of Celnovte Reagents and Instruments.
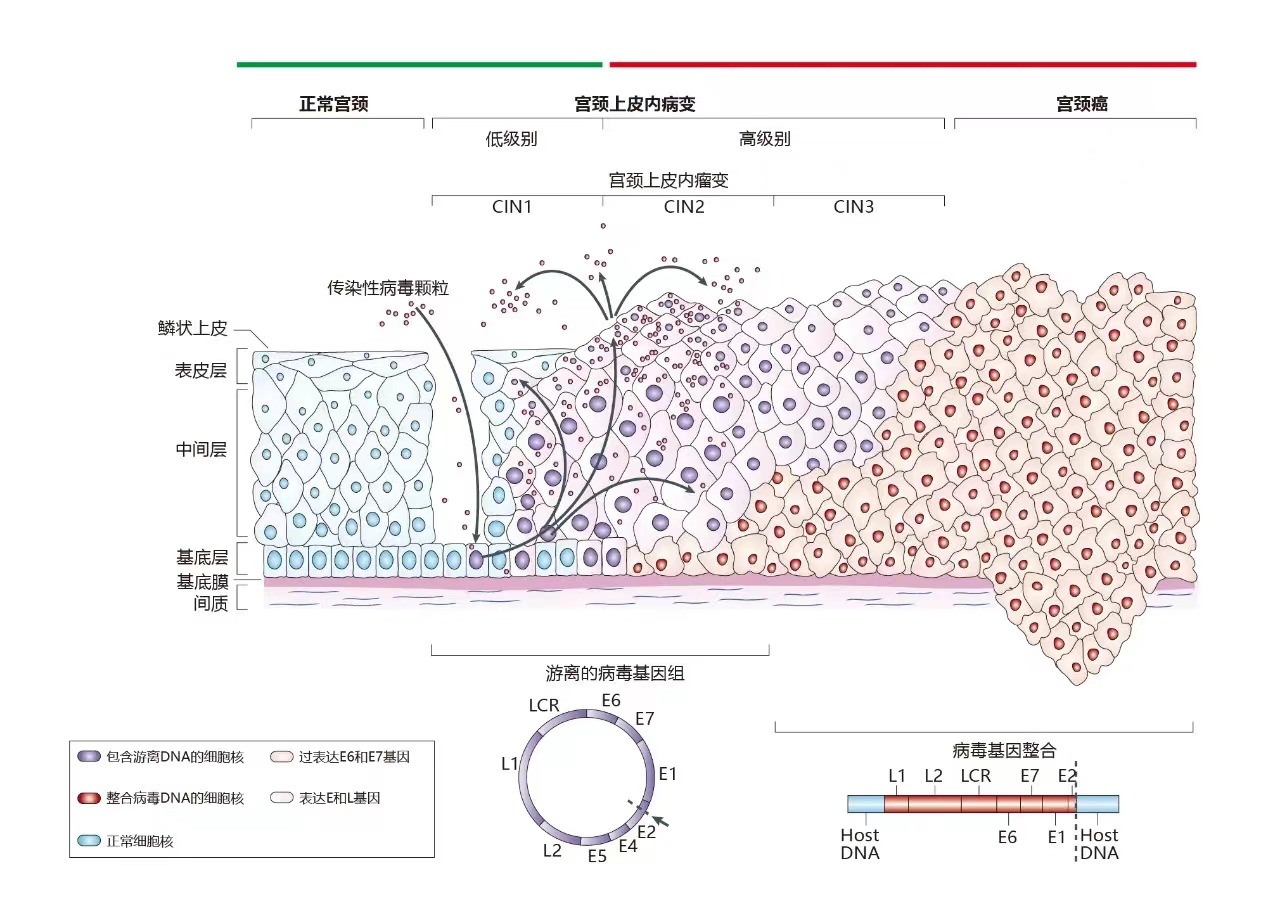
International guidelines for cervical cancer screening, influenced by WHO and national cancer agencies, emphasize early detection and treatment of pre-cancerous conditions.
In the ongoing fight against cervical cancer, HPV DNA test offers significant advantages in early cervical cancer screening than Pap smears, especially in terms of sensitivity and early detection, however, A positive HPV DNA test does not necessarily mean a person has cervical cancer and may cause anxiety.
The purpose of HPV detection is not merely to identify HPV infections, but to detect CIN2+ lesions or assess the risk of progression to cancer.
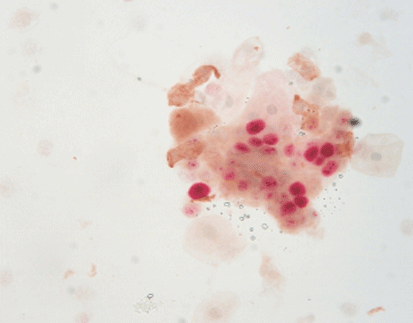
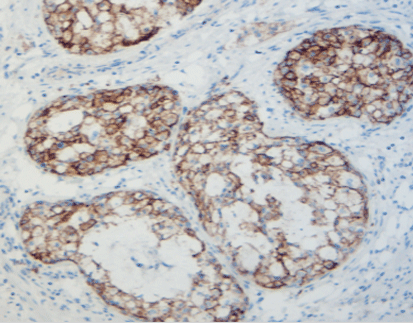
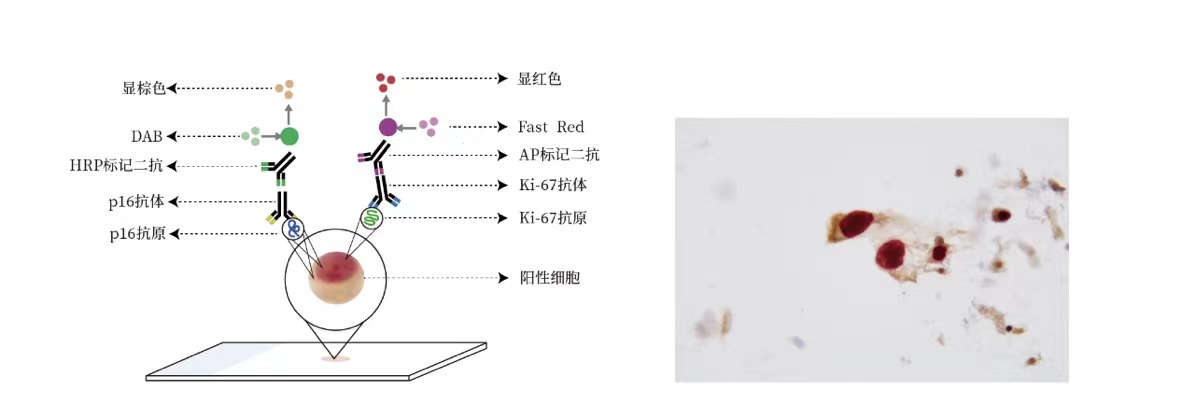
p16/Ki-67 dual staining is a valuable tool in the early detection and management of cervical cancer. Its ability to accurately identify high-risk lesions aids in making informed decisions regarding patient care, ultimately contributing to better outcomes in cervical cancer treatment and prevention.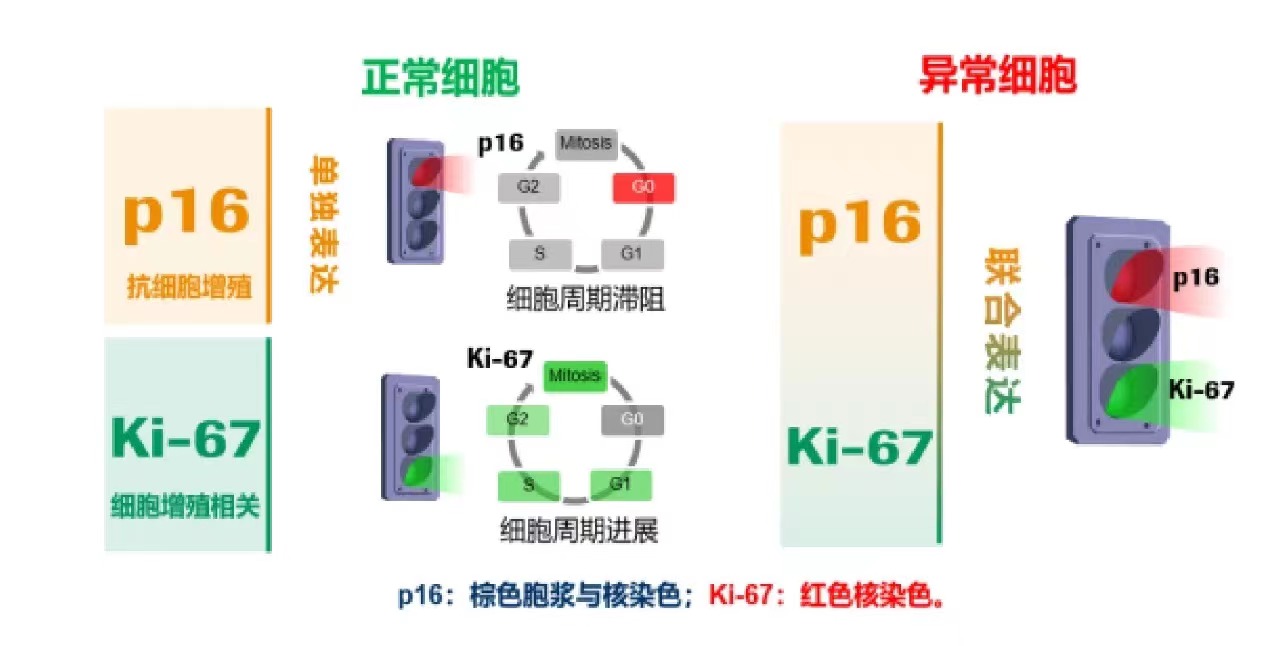
The American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines incorporate biomarkers like p16/Ki-67 to enhance the management of cervical cancer screening and treatment.
Celnovte Dual Staining Kit Features
 0
0