Key Elements That Shape Accuracy in Molecular Diagnostics

By admin

Anyone who works in a hospital lab knows that molecular diagnostics has changed everything about how we treat patients. But real success isn’t counted in published papers or fancy journal scores. It shows up when a test result arrives fast, spots the problem correctly, and helps the oncologist pick the exact drug that will work for that one person on the exam table. A tiny slip in the lab can mean the difference between the right therapy and the wrong one. That’s why accuracy isn’t just a nice feature in this field—it’s the whole game.
This article walks through the everyday factors that decide whether a molecular test can be trusted: the reagents on the bench, the staining methods pathologists use, and the machines that run the slides. Along the way, you’ll see how Celnovte keeps raising the standard for labs in hospitals and reference centers worldwide.
The True “Impact Factor” of Precision Medicine
Beyond Bibliometrics: Clinical Impact in Diagnostics
Molecular tests look straight at DNA or RNA sequences—whether it’s a cancer mutation hiding in a biopsy or the genetic signature of a virus in a swab. Doctors often spot trouble months or even years before the patient feels anything wrong. Catching it that early usually gives treatment a much better shot at working.
On top of that, these tests let doctors choose medicines that match the patient’s own biology instead of guessing. Fewer nasty side effects, less time lost on drugs that won’t help—that’s what personalized care really looks like, and it all rests on getting the test result right the first time.
Defining Accuracy: The Bedrock of Personalized Therapy
At the bench, accuracy comes down to two numbers everybody watches: sensitivity (how well the test finds the bad stuff when it’s really there) and specificity (how well it says “nothing to see here” only when that’s true). A false negative can mean a missed chance at curative therapy. A false positive can send someone for extra scans, biopsies, or treatments they never needed.
Good molecular assays regularly clear 95 % on both measures. Celnovte’s RT-PCR kits, for instance, consistently hit sensitivity and specificity above 95 % when hospitals run their daily controls.
Foundational Elements: High-Fidelity Detection Systems
Clean, reliable staining starts with reagents and methods that simply work better than the rest. Celnovte has built its name supplying pathology departments with IHC, CISH, and FISH tools that pathologists reach for first when the case is tough.
Maximizing Signal Sensitivity in Immuno-Oncology
IHC is still the workhorse for confirming most cancers under the microscope. To read a slide confidently, the brown or red signal has to pop against a quiet background—no muddy haze, no blank spots where tumor cells should light up.
Ask any pathologist who has switched to Celnovte’s MicroStacker™ Detection systems, and they’ll tell you the stains look sharper and stronger. The latest model, MicroStacker™ Ultra, that came out in 2023, pushes performance another notch higher.
Product Features & Advantages:
-
Advanced Polymer Technology: A special polymer backbone carries more enzyme molecules right to the target, giving a much brighter signal.
-
Reduced Ambiguity: Strong target staining plus almost no background means far fewer slides land in the “maybe” pile. Pathologists miss fewer positives and don’t chase false ones.
-
Clinical Validation: Over 47 of Celnovte’s in-house cloned primary antibodies have scored “optimal” or “good” on NordiQC external runs. That independent report card tells labs everywhere the reagents will behave the same way on their own benches.
![[RX] MicroStacker™ Mouse-on-Mouse HRP Polymer](http://www.celnovte.com/wp-content/uploads/2025/12/RX-MicroStacker™-Mouse-on-Mouse-HRP-Polymer.jpg)
The Essential Role of Workflow Automation
Even perfect reagents can give spotty results if one tech rinses five seconds longer than the next. Automation locks every step in place so the staining looks the same whether it’s slide one or slide one hundred.
Eliminating Variability through Standardized Instrumentation
Automated stainers handle the timing, temperature, and reagent volumes exactly the same way every run. The pathologist reading the slide knows any color differences come from the tissue biology, not from someone hurrying through a wash.
Celnovte’s fully automatic stainer offers high throughput and consistent results. The CNT 360 runs complete IHC and ISH protocols without anyone opening the door mid-cycle.
Product Features & Advantages:
-
All-in-One Workflow: One platform handles routine IHC, special stains, and in situ hybridization, saving bench space and staff training time.
-
Unparalleled Efficiency: PolyStacker™ technology built into the system can finish frozen-section IHC in as little as 10 minutes—critical when the surgeon is waiting for margins.
-
Seamless Integration: Over 800 Celnovte automated stainers have been running daily in hospitals since 2018, proof that the machines hold up under real-world pressure and keep turning out uniform slides.
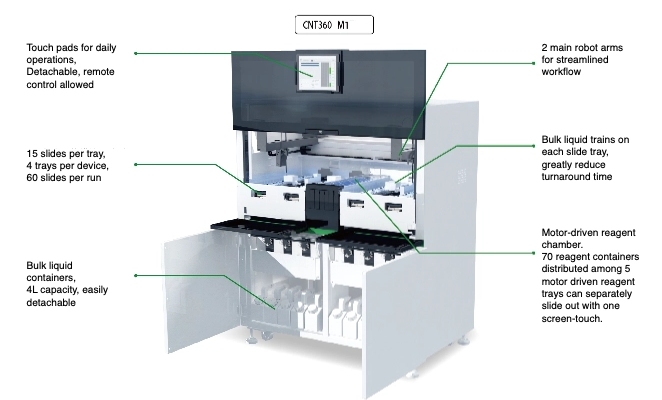
Quantifying Efficiency: Speed, Consistency, and Clinical Confidence
Faster, more consistent results mean oncologists get answers while the patient is still in the clinic or the OR. Pair the CNT 360 with Celnovte’s ready-to-use kits and the whole line—from dewax to counterstain—stays predictable even on the busiest days.
Celnovte’s Commitment to Diagnostic Excellence
Celnovte Biotech started in 2010 with a clear focus: build tools that pathologists actually need. Reagent development happens in Rockville, Maryland; large-scale manufacturing runs in modern plants in China. Today, our products are used in more than 2300 major hospitals across China and in over 40 countries.
Quality Assurance: Compliance and External Validation
Every factory meets NMPA & GMP standards and carries ISO13485, ISO9001, FDA registration, and CE IVDR certification. Those stamps mean the sensitivity and specificity numbers printed on a MicroStacker™ box or RT-PCR kit insert are backed by tight manufacturing control, no matter where the lab is located.
Conclusion: Sustaining Accuracy for Patient Outcomes
When all is said and done, the only impact factor that counts in molecular diagnostics is whether the result helps the doctor standing in front of a worried patient. By pairing sharp tools like MicroStacker™ Ultra from rock-steady automation on the CNT 360, labs can hand clinicians the trustworthy answers personalized medicine needs. Celnovte keeps pushing forward so that life-changing accuracy isn’t left to chance—it’s baked into every reagent bottle and instrument they ship.
FAQ
Q: What is molecular diagnostics used for?
A: It spots disease by reading DNA or RNA directly—cancer mutations, infections, inherited conditions—often long before symptoms appear.
Q: How accurate are Celnovte’s detection systems?
A: They’re built to hit the mark. RT-PCR kits regularly show sensitivity and specificity above 95 %, and many primary antibodies have earned top NordiQC scores in external quality runs.
Q: What is the primary advantage of automated stainers like the CNT 360?
A: They remove day-to-day human variation, cut turnaround time, and keep every slide looking the same even when the lab is slammed.
Q: Does Celnovte provide solutions for genetic disorder screening?
A: Yes. The HerediScreen Genetic Test Kit is a non-invasive option that checks for common inherited conditions.
RELATED PRODUCTS






