How to Interpret IHC Positive Results: A Guide for General Readers
2025-08-21
By admin
Understanding IHC and Its Diagnostic Role
What Is Immunohistochemistry (IHC)
Immunohistochemistry, or IHC, is a laboratory technique that combines anatomical, immunological, and biochemical methods to identify specific antigens in tissue sections using antibodies. Immunohistochemistry is a valuable tool in pathology because it facilitates the identification of the presence and distribution of proteins in tissue specimens, which is helpful in disease diagnosis and classification.
Why IHC Testing Is Used in Pathology
IHC analysis is widely applied for the diagnosis of various cancers, determination of tumor origin, prognosis assessment, and decision-making in therapy. It allows pathologists to visualize protein expression patterns directly within the framework of intact tissue architecture. With particular interest in the fields of early screening, precise diagnosis, drug companion, and monitoring of tumor treatment, Celnovte focuses on the setup of a whole industrial chain covering research and development, production, sales, and service.
What a Positive IHC Result Indicates
Definition of “Positive” in an IHC Context
“IHC positive” specifies the identification of a specific protein or antigen in the tissue sample through staining. A positive finding indicates the target antigen is present at detectable levels and has bound to the utilized antibody upon testing.
Why Protein Expression Affects Diagnosis
A positive IHC result may rule in or rule out a few diagnoses based on protein expression patterns. For example, estrogen receptor (ER) positivity of breast carcinoma confirms hormone-dependent tumor growth and sensitivity to hormonal therapy. Up to now, it has made nearly 80 antibodies, of which antibodies such as ER, PR, and HER2PD-L1 that can be used to positively guide treatment protocols have been of good quality.
Factors That Influence IHC Results
Sample Quality and Preparation
Tissue fixation time, type of fixative used, and processing steps significantly impact antigen preservation. Our fixative has been verified by a large number of clinical practices to ensure the accuracy of antibody staining results. Using suboptimal fixatives may lead to weak or false-negative staining.
Antibody Specificity and Sensitivity
High-quality primary antibodies with strong specificity are essential for accurate target detection. Celnovte Antibody R&D Center specializes in the development of IVD-grade immunohistochemistry primary antibodies. Customized antibody development ensures that antibodies are tailored for specific diagnostic needs.
Importance of Standardized Protocols
Standardization minimizes variability between labs and technicians. For technicians who are proficient in immunohistochemical operation steps, the staining results are definitely consistent. Automation further enhances reproducibility across multiple samples.
Key Celnovte Products Supporting Accurate IHC
Celnovte CNT300 Full Automatic Multiplex IHC Stainer: Automated Precision Staining
The CNT300 is a fully automated immunohistochemistry stainer with a throughput of 60 slides per run and completes staining within 2.5 hours. It supports advanced applications such as chromogenic in situ hybridization (EBER), multi-color immunohistochemistry, and p16/Ki-67 double staining. This automation reduces human error while increasing efficiency—ideal for high-throughput labs.
Celnovte Primary Antibodies Series: Reliable Target Detection
Celnovte offers over 460 primary antibodies validated for diagnostic use. These include critical markers like HER2, ER, PR, PD-L1—all essential for guiding cancer treatment decisions. ER obtained a Class I registration certificate approved by NMPA in 2022. The antibodies are produced using murine monoclonal technology or human monoclonal methods via customized development platforms.
Common Types of Positive IHC Markers
Hormone Receptors (ER, PR) in Breast Cancer
Positive ER or PR results suggest that breast cancer cells rely on hormones for growth. These patients often benefit from endocrine therapies like tamoxifen or aromatase inhibitors. Antibodies such as ER and PR developed by Celnovte have passed NordiOC quality control evaluation activities.
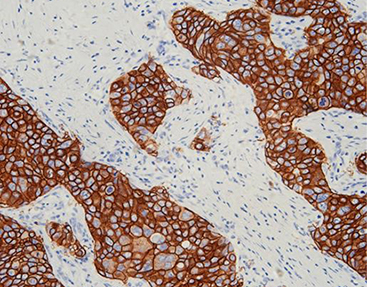
HER2 Positivity and Its Implications
HER2 positivity indicates aggressive tumor behavior but also identifies patients who may benefit from targeted therapies like trastuzumab. Accurate detection is vital; hence, high-specificity antibodies from Celnovte ensure reliable HER2 assessment.
PD-L1 Expression in Immunotherapy
PD-L1 positivity helps determine eligibility for immune checkpoint inhibitors in cancers like NSCLC or melanoma. Celnovte’s PD-L1 antibody is designed with high sensitivity for consistent results across various specimen types.
Interpreting Staining Patterns and Intensity
Membranous, Cytoplasmic, and Nuclear Staining Differences
The location of staining helps interpret protein function:
- Membranous staining (e.g., HER2)
- Nuclear staining (e.g., ER)
- Cytoplasmic staining (e.g., cytokeratin)
Understanding these patterns aids pathologists in differentiating between benign and malignant lesions.
Scoring Systems Used by Pathologists
Scoring combines intensity (weak/moderate/strong) with percentage positivity. For example:
- HER2 scoring uses a 0–3+ scale
- ER/PR scoring involves the Allred score
Automated platforms like CNT300 standardize stain intensity across samples for more objective scoring.
Reducing False Positives in IHC Testing
Importance of Reagent Quality and Controls
False positives can occur due to non-specific binding or cross-reactivity. Using validated reagents minimizes this risk.
Celnovte currently has more than 200 immunohistochemistry quality control products, ensuring consistent performance across batches.
Breast lumps can be found with internal controls, allowing real-time validation within each slide.
FAQ
Q: What does an “IHC positive” result mean?
A: It means that the targeted protein was detected in your tissue sample using specific antibodies during testing—indicating its presence at a diagnostically relevant level.
Q: Can frozen section results differ from paraffin section results?
A: Yes. There are positive tissues in the frozen section, and there may be none or vice versa after paraffinization. This discrepancy often arises due to sampling differences or tissue size limitations.
Q: Are customized antibodies better than commercial off-the-shelf ones?
A: Customized antibodies offer higher specificity tailored to your research or diagnostic needs. Celnovte has a professional antibody development team that supports customized development services for murine monoclonal antibodies and human monoclonal antibodies.
Q: Why is intraoperative immunohistochemistry needed if HE staining was already done?
A: HE provides morphology only; some tumors require additional markers for accurate diagnosis. Intraoperative immunohistochemistry can improve the diagnostic accuracy of pathologists and reduce delayed diagnosis, helping surgeons make informed decisions during surgery.