Comprehensive Mismatch Repair (MMR) Solution
2025-12-22
By admin
MMR IHC Detection Kit for dMMR / MSI-H Tumors – A Complete and Standardized Immunohistochemistry Solution
Accurate MMR immunohistochemistry (IHC) testing plays a critical role in modern oncology, supporting cancer diagnosis, prognosis assessment, and therapeutic decision-making.
Celnovte Biotechnology provides a comprehensive, end-to-end MMR IHC detection solution designed to deliver consistent staining quality, high diagnostic confidence, and robust laboratory standardization.
What Is the Mismatch Repair (MMR) System?
The Mismatch Repair (MMR) system is an essential DNA repair mechanism that corrects base-pair mismatches arising during DNA replication. By maintaining genomic integrity, the MMR pathway plays a vital role in preventing mutation accumulation and tumorigenesis.
The core MMR proteins include MLH1, MSH2, MSH6, and PMS2, which belong to the MutL and MutS protein families.
Loss of nuclear expression of any of these proteins on IHC indicates deficient mismatch repair (dMMR), a molecular phenotype frequently associated with microsatellite instability-high (MSI-H) tumors.
Clinical Significance of MMR Protein IHC Testing
1. Lynch Syndrome Screening
MMR IHC is widely used as a first-line screening tool for Lynch syndrome, an autosomal dominant hereditary cancer syndrome linked to colorectal, endometrial, gastric, and ovarian cancers.
More than 90% of Lynch syndrome cases demonstrate a dMMR / MSI-H phenotype, making MMR IHC a highly effective screening approach.
2. Prognostic Stratification
In stage II colorectal cancer, tumors with dMMR / MSI-H status are associated with lower recurrence risk and improved overall survival compared with MMR-proficient tumors.
In endometrial carcinoma, TCGA molecular classification further refines prognosis:
-
POLE-ultramutated: Best prognosis
-
MSI-H (dMMR): Favorable prognosis
-
Copy-number low (CN-L): Intermediate prognosis
-
Copy-number high (CN-H): Poor prognosis
3. Chemotherapy Response Prediction
Patients with dMMR / MSI-H colorectal cancer typically show limited benefit from single-agent fluoropyrimidine-based adjuvant chemotherapy, making accurate MMR status determination essential for optimal treatment planning.
4. Immunotherapy Biomarker
dMMR / MSI-H tumors demonstrate a high response rate to immune checkpoint inhibitors (ICIs).
As a result, MMR IHC and MSI testing are recommended predictive biomarkers in multiple international oncology and pathology guidelines.
Common Challenges in MMR IHC Interpretation
Despite its clinical importance, MMR IHC interpretation can be technically challenging due to:
-
Cytoplasmic or non-specific staining
-
Weak or absent internal control staining
-
Tumor heterogeneity
-
Effects of neoadjuvant chemoradiotherapy
-
Rare or atypical staining patterns
-
Variability in tissue type, fixation, and processing
To ensure reliable and reproducible results, standardized reagents, optimized detection systems, and validated staining protocols are essential.
Celnovte’s Complete MMR IHC Detection Solution
Celnovte offers a fully optimized, end-to-end MMR IHC detection kit that integrates primary antibodies, detection chemistry, and standardized protocols to support consistent and high-quality diagnostics across laboratories.
Key Features of the MMR IHC Kit
High-Sensitivity Primary Antibody Panel
-
Validated monoclonal antibodies for MLH1, MSH2, MSH6, and PMS2
-
Excellent batch-to-batch consistency
-
Uniform nuclear staining with minimal background
Specialized Polymer Detection System
-
High-sensitivity polymer-based secondary antibody detection
-
Specifically optimized for MMR IHC applications
-
Strong signal amplification with low non-specific staining
Standardized & Validated Protocols
-
Antibody-specific antigen retrieval and incubation conditions
-
Improved reproducibility and inter-laboratory consistency
-
Reduced interpretation variability
Automation-Ready IHC Workflow
-
Compatible with automated IHC staining platforms
-
Optimized performance from reagents to instrumentation
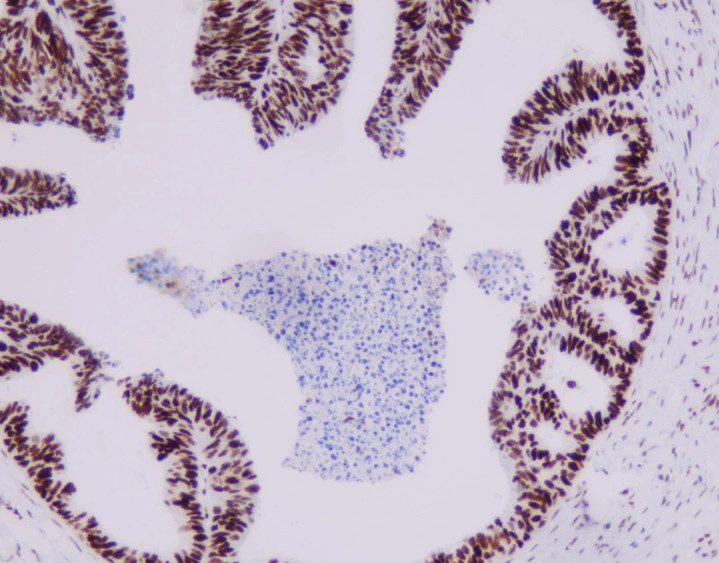
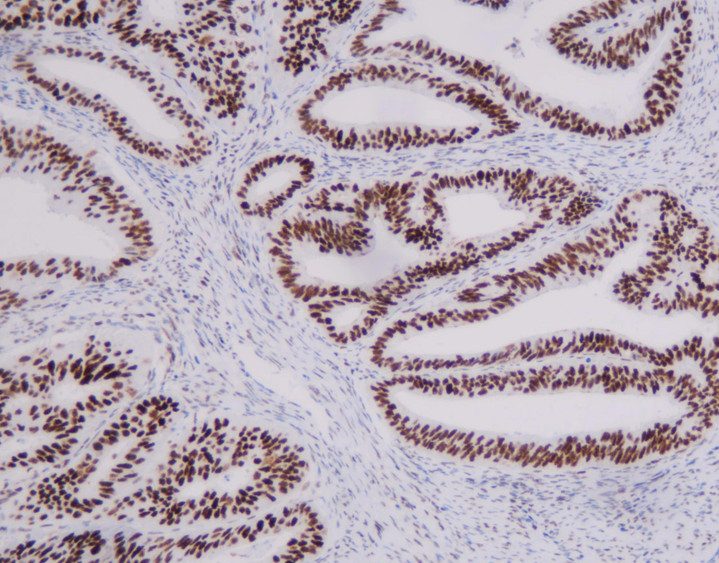
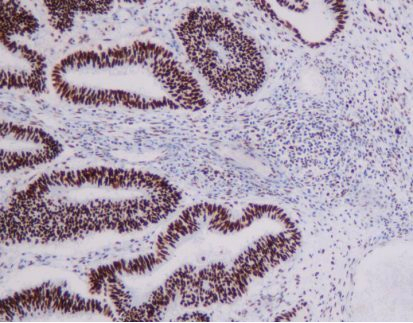
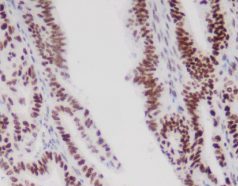
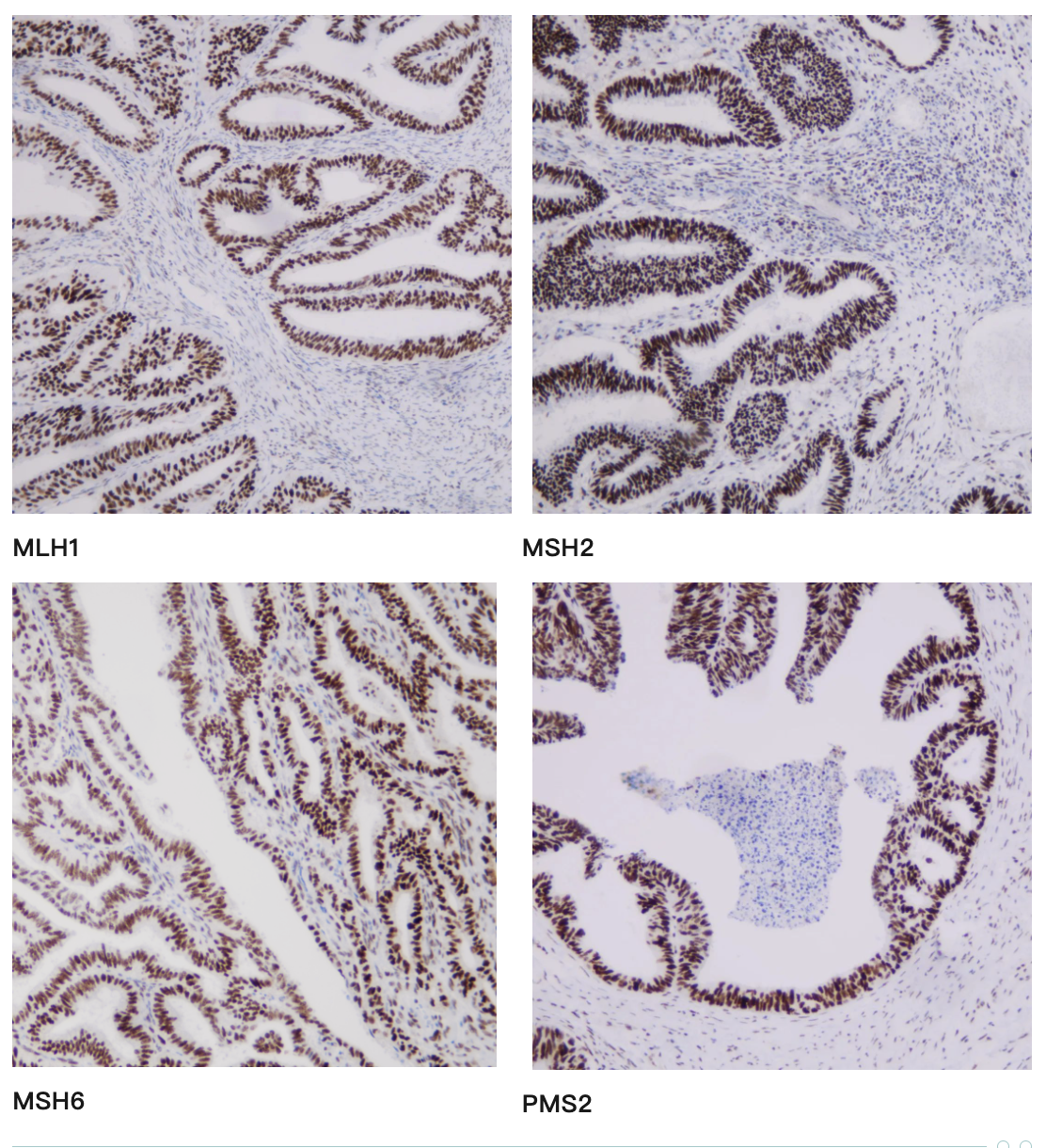
Representative MMR IHC Staining Results
-
MLH1: Clear nuclear staining with minimal background
-
MSH2: Strong, specific nuclear signal in tumor and control cells
-
MSH6: High sensitivity with excellent tissue morphology
-
PMS2: Reliable nuclear localization with intact internal controls
Why Choose Celnovte’s MMR IHC Detection Kit?
Celnovte’s MMR solution combines high-performance antibodies, optimized detection chemistry, validated protocols, and automation compatibility to address real-world diagnostic challenges in MMR testing.
Improve MMR testing accuracy, reproducibility, and clinical confidence with a fully standardized IHC solution.
MMR Solution Highlights
-
Clinically relevant antibodies: MLH1, MSH2, MSH6, PMS2
-
Robust polymer detection: Strong and consistent signal amplification
-
Standardized protocols: Designed for routine clinical diagnostics
Conclusion
Celnovte’s MMR IHC detection solution enables accurate identification of dMMR / MSI-H tumors, supporting personalized oncology, immunotherapy selection, and standardized pathology workflows worldwide.
Frequently Asked Questions (FAQ)
What does MMR mean in pathology?
MMR refers to the DNA mismatch repair system responsible for correcting replication errors and maintaining genomic stability.
Why is MMR IHC testing important?
MMR IHC supports Lynch syndrome screening, prognostic evaluation, chemotherapy selection, and prediction of immunotherapy response.
How does this MMR IHC kit improve accuracy?
By combining high-quality monoclonal antibodies, optimized polymer detection chemistry, and standardized staining protocols validated for clinical use.