PRODUCT CATEGORY
PRODUCT CATEGORY
CONTACT US
Phone
Email
Address
6 Cuizhu St, Zhong Yuan Qu, Zheng Zhou Shi, He Nan Sheng, China, 450001
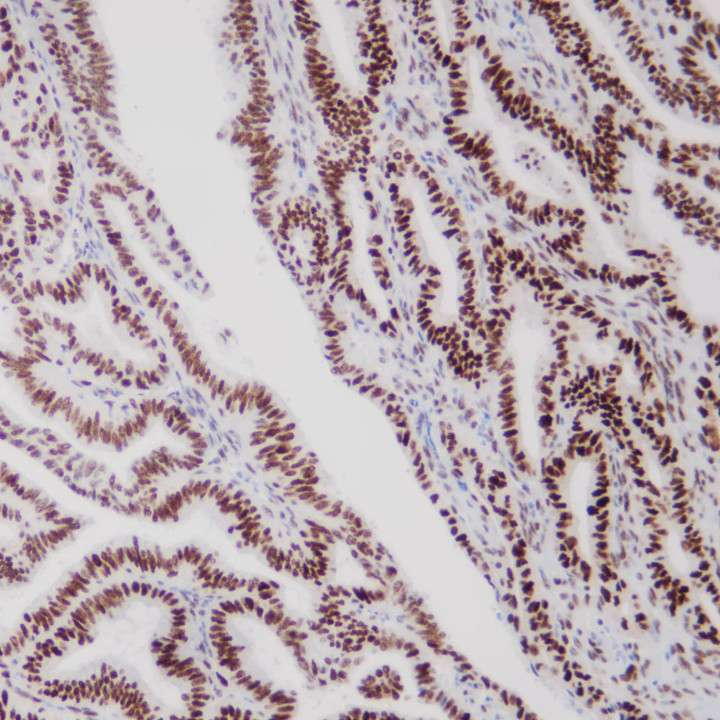
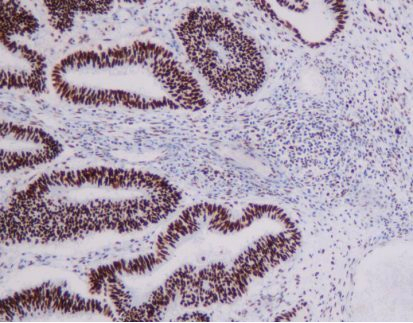
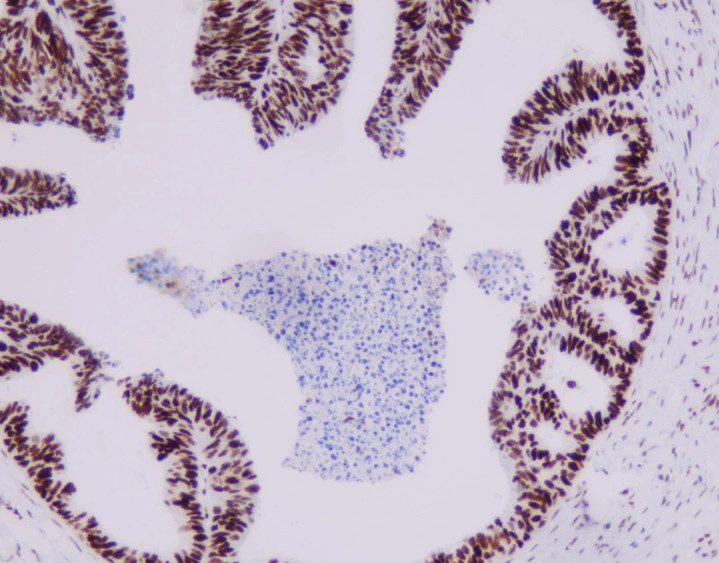
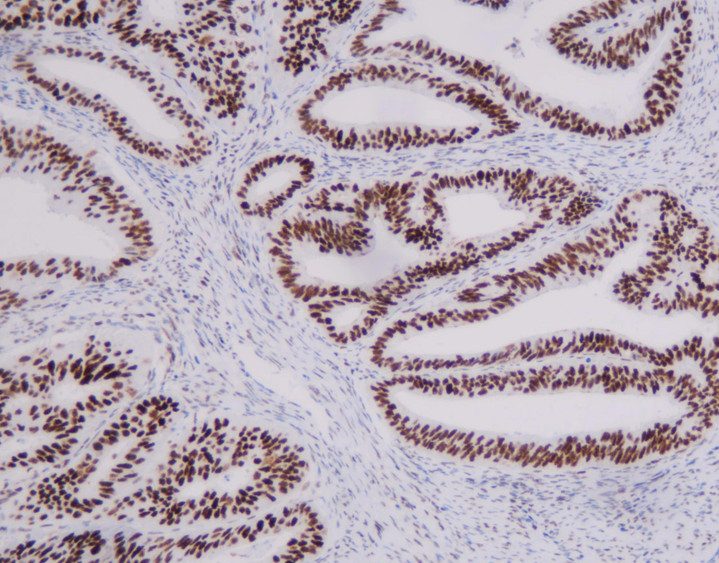
MMR panel | MSH6 (C5D11), MMab
MSH6 is an important member of the mismatch repair family and is positioned on chromosome 2 P arm 16 point. The DNA length is 23806BP, while the main function is to correct base pair mismatch and small fragment insertion and deficiency. When DNA is mismatched, MSH6 protein and MSH2 protein combine to produce A MutS-α heterodimer that can be identified for the mismatch site. This The heterodimer is combined with the mismatch site and initiates the mismatch repair process, thus ensuring the stability of the microsatellite.
Product Features
[Intended Use]
This antibody is intended for use in Immunohistochemical applications on formalin-fixed paraffin-embedded tissues (FFPE). The clinical interpretation of any staining or its absence should be complemented by morphological studies using proper controls and should be evaluated within the context of the patient’s clinical history and other diagnostic tests by a qualified pathologist.
[Specifications]
| Product Name | MSH6 (C5D11), MMab |
| Catalog No. | CMM-0205 |
| Intended Use | IVD, RUO |
| Species Reactivity | Human; others not tested |
| Cellular Localization | Nuclear |
| Antibody Type | Mouse Monoclonal |
| Clone | C5D11 |
| Format and Volume | Ready-to-use: 1.5mL, 7.5mL Concentrated: 0.1mL, 0.2mL and 1mL |
[Datasheets & SDS]
| IVD Datasheet (IFU) | ↕️ Download |
| RUO Datasheet (IFU) | ↕️ Download |
| SDS sheet | check with sales |
[Storage and Validity]
Store at 2~8°C. Avoid freezing.
Maintain temperature below room temperature during transport, ensuring it does not exceed one week.
[Protocol Recommendations]
This reagent has been optimized on the fully automatic immunohistochemical stainer and does not require reconstitution, mixing, dilution or titration.
For detailed operating parameters, please refer to the operating manual of the fully automatic immunohistochemical stainer.
1.Test Procedure:
1.1 Incubation with peroxidase block quenches the activity of endogenous peroxidase.
1.2 Use MSH6 to incubate.
1.3 Linker , rabbit-anti-mouse incubation.
1.4 Use polymer for incubation, and bind the reagent to linker , rabbit-anti-mouse .
1.5 Incubate the antigen-antibody complex with DAB, and finally appear in the form of a brown precipitate, which can be observed under a light microscope.
1.6 Use DAB Enhancer for incubation to enhance color intensity.
1.7 Incubate with hematoxylin and counterstain the sections.
1.8 Use bluing reagent to incubate and blue hematoxylin to enhance the contrast of the staining results.
2.Quality Control
2.1 Positive Control
Positive controls serve as an indication of correct tissue preparation and appropriate staining techniques. Each staining should include a positive pair of photos for comparison under the same test conditions. Known positive tissue controls should only be used to monitor the correct performance of procedures and testing of reagents and are not intended to aid in describing a definitive diagnosis of a patient sample. If the positive tissue control fails to show appropriate positive staining, the results of this batch of experimental test samples should be considered invalid.
2.2 Blank Control
Each staining should include a blank control reagent under the same test conditions for comparison. Blank control reagents are used to stain tissue sections instead of antibodies to judge non-specific staining and provide a better explanation for antigen site-specific staining.
[Reference]
1. Shia J. J Mol Diagn. 2008;10(4):293–300.
2. Bellizzi AM. Surg Pathol Clin. 2017;10(4):843–868.
3. Park JY et al. Pathol Res Pract. 2012;208(7):428–433.
4. Hendricks LA et al. J Pathol. 2004;204(1):28–35.