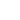 PRODUCT CATEGORY
PRODUCT CATEGORY
CONTACT US
 Phone
Phone
 Email
Email
 Address
Address
ZhengZhou (HQ), Shenzhen (R&D Center), Suzhou (R&D Center), China
ALK Breakapart Detection Kit
Clinic Signification:
1.About 60%-85% of cases of anaplastic large cell lymphoma (ALCL) express anaplastic lymphoma kinase (ALK) fusion protein, which is caused by the breakapart of the ALK gene locus on chromosome 2 and fusion with other genes. The 5-year survival rate of patients with ALK gene fused with other genes (ALK positive) is much better than that of ALK negative patients (about 70% vs 30%), and the overall survival rate is much better than the latter.
2.Chromosomal translocation and ALK expression have been prescribed by WHO as one of the clinical diagnostic indicators of ALCL, and are the targets of the drug crizotinib.
Contrast
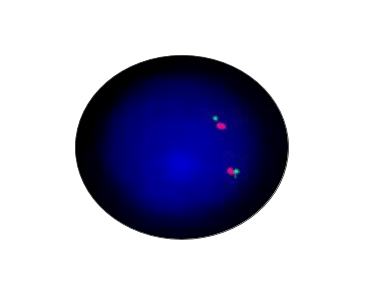 ALK Normal
ALK Normal
ALK Breakapart
More Info
Probe Description: ALK
Cat.No.: CF1010
Specification: 10 tests/box, 20 tests/box
Related Product











 Chat
Chat

 message
message

 Quote
Quote