Theoretical Aspects of Claudin18.2 Immunohistochemistry Evaluation in Gastric/Gastroesophageal Junction Adenocarcinoma

By admin
Gastric cancer ranks among the most widespread harmful tumors globally, with elevated occurrence and death rates, placing a substantial strain on numerous households. With progress in cancer research and molecular studies, the management of advanced gastric cancer has transitioned from a uniform chemotherapy method to tailored treatments targeting patients’ unique molecular traits. The SPOTLIGHT and GLOW studies have repeatedly shown the promise of Claudin18.2 as a rising treatment focus in gastric cancer, making its consistent detection a subject of considerable medical attention.
Current Status of Biomarker Testing in Advanced or Metastatic Gastric/Gastroesophageal Junction Adenocarcinoma
As our grasp of the molecular aspects of gastric/gastroesophageal junction adenocarcinoma grows, targeted treatments have become more common. Many gastric cancer patients receive a diagnosis at a locally progressed or metastatic phase, where surgery alone is no longer suitable. Tissue-based biomarker analysis has become essential for customized systemic therapy.
HER2 was the initial biomarker regularly included in gastric cancer pathology assessments, a practice started over ten years ago. Recently, with immune checkpoint inhibitors confirmed as a primary treatment, PD-L1 expression levels have also become a vital part of diagnostic reviews for advanced gastric cancer. Moreover, national and global guidelines suggest checking DNA mismatch repair (MMR) protein status or microsatellite instability (MSI) before beginning therapy. Thus, HER2, PD-L1, and dMMR/MSI-H are key biomarkers that must be tested in patients with advanced metastatic gastric cancer before initial therapy. The recent clinical endorsement of Claudin18.2-targeted medications is likely to encourage broad adoption of Claudin18.2 testing. In the coming years, additional biomarkers, such as FGFR2b, are expected to be integrated into standard pathology assessments, further influencing clinical approaches.
Claudin18.2 as a Promising Target in Gastric Cancer
Claudin18.2 is a member of the Claudin (CLDN) protein group, first identified in 1998. This group creates tight connections between neighboring cells, controlling substance movement. Claudin18.2 is among the most carefully examined tight junction proteins.
Molecular Biology Characteristics
The CLDN18 gene located at chromosome 3q22 encodes the Claudin18 protein with two outer loops, four crossing domains, and an inner domain. Alternative splicing of the CLDN18 gene gives rise to two isoforms: CLDN18.1 in lung tissue and Claudin18.2 in gastric tissue. Claudin18.2 is a crucial member of epithelial tight junctions, responsible for cell structure, barrier properties, and acid resistance. In 2008, Professor Sahin showed that in healthy gastric tissue, Claudin18.2 occurs just within gastric mucosal lining cells and is hidden. But in cancer cells, tumor exposure to cause cellular structure breakdown brings Claudin18.2 markers to the surface for attachment of targeted drugs. This feature identifies Claudin18.2 as a potential treatment target in gastric cancer.
Claudin18.2 Expression in Gastric Cancer
Due to differences in antibodies, positivity standards, staining evaluation methods, tumor type/location, and sample kinds (biopsy vs. surgical), reports on Claudin18.2 positivity rates have varied. The largest dataset so far shows that 38.4% (1730/4507) of gastric/gastroesophageal junction adenocarcinomas display moderate-to-strong Claudin18.2 membrane staining in at least 75% of tumor cells. In metastatic gastric cancer, Claudin18.2 expression remains highly similar between matched primary and metastatic sites, with no notable link to tumor stage. From a treatment viewpoint, Claudin18.2 expression has minimal overlap with other biomarkers like HER2, PD-L1, and MMR proteins.
Clinical Features of Claudin18.2-Positive Gastric Cancer
Several past studies suggest that Claudin18.2 positivity is more common in poorly cohesive or diffuse-type gastric cancers, though this link has not been universally supported. A specific CLDN18-ARHGAP fusion mutation, found in about 15% of gastric cancers, has been noted. This fusion is tied to early-onset, diffuse-type gastric cancer, advanced stages, and worse outcomes, often paired with Claudin18.2 overexpression.
Claudin18.2-Targeted Therapies Reach the Clinic
Zolbetuximab (VYLOY), the first Claudin18.2-targeted monoclonal antibody, is a human-mouse hybrid IgG1 antibody that specifically attaches to Claudin18.2, triggering tumor cell death through antibody-dependent cellular toxicity and complement-dependent toxicity. During zolbetuximab’s clinical trials, the definition of Claudin18.2 positivity shifted. Initially, phase I trials used “any tumor cell membrane expression (regardless of strength).” Later, the phase III SPOTLIGHT and GLOW trials confirmed it as “moderate-to-strong membrane staining in ≥75% of tumor cells.” Both trials showed the better efficacy and safe tolerability of zolbetuximab when administered with chemotherapy in Claudin18.2-positive, HER2-negative gastric cancer. The treatment of Claudin18.2-positive gastric cancer is advancing rapidly, and new approaches like bispecific and trispecific antibodies are set to increase antitumor activity.
Enhancing the Standardization of Claudin18.2 Detection and Interpretation
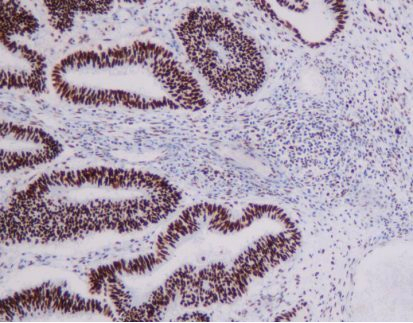
Immunohistochemistry (IHC) is the test of choice for confirmation of Claudin18.2 expression, but inconsistencies in antibodies and scoring systems have resulted in conflicting findings among studies. Standard IHC staining and assessment guidelines are key to producing reliable prospective data and to identifying patients who are treatable.
Selection of Detection Antibodies and Platforms
For Claudin18.2 IHC, the Clone 43-14A antibody (Ganymed, Germany) is commonly employed. This antibody targets the C-terminus of claudin-18 and, while not exclusive to the 18.2 variant, provides reliable results. A comparison study testing three CLDN18 antibodies and IHC systems found that the Ventana CLDN18 (43-14A) platform achieved at least 95% performance (accuracy, sensitivity, and specificity) with strong consistency across 27 labs.
Basic Requirements for Claudin18.2 Expression Assessment
1. Sample Selection:
- Test at least six sites from primary tumor biopsy samples.
- Evaluate only invasive adenocarcinoma; exclude non-invasive or dysplastic cells.
- Ensure samples contain at least 50 malignant tumor cells.
2. Analysis Requirements:
- Perform Claudin18.2 staining on routinely processed, formalin-fixed, paraffin-embedded tissue.
- Unstained slides should maintain antigen stability for at least 45 days, with potential extensions based on lab-specific validation.
- As CLDN18.1 expression in gastric tissue is negligible, subtype-specific or pan-claudin-18 antibodies yield comparable results.
3. Staining Evaluation:
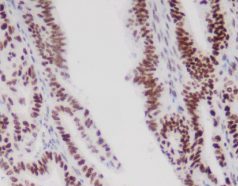
- Classify Claudin18.2 expression by staining intensity: 0 (no membrane or cytoplasmic reaction), 1+ (weak membrane or cytoplasmic reactivity), 2+ (moderate membrane or cytoplasmic reactivity), 3+ (strong membrane or cytoplasmic reactivity).
- Begin evaluation under low magnification (x5 objective) to assess overall staining and expression heterogeneity.
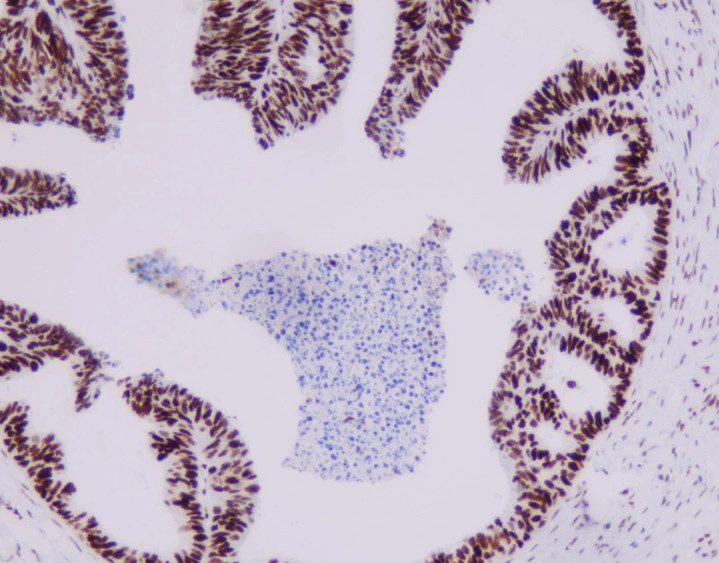
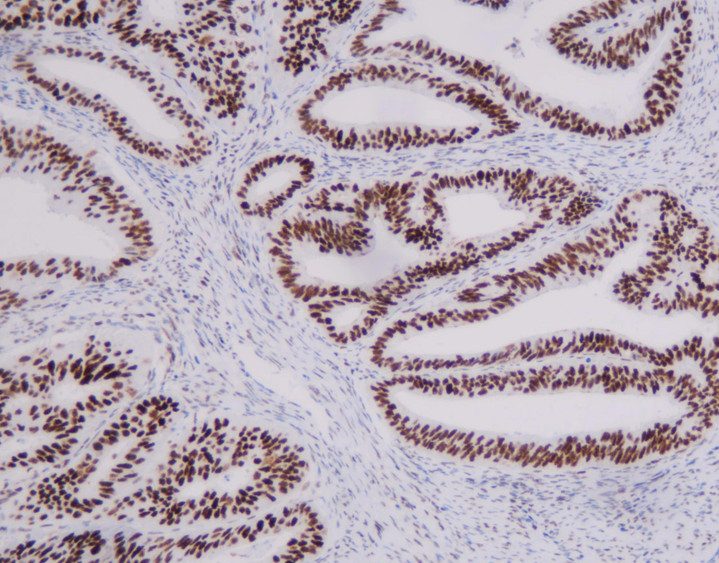
- Based on SPOTLIGHT and GLOW studies, Claudin18.2 positivity is defined as 2+/3+ membrane staining in ≥75% of tumor cells. When 2+/3+ staining ranges between 60% and 80%, a second pathologist should re-evaluate.
- Non-tumorous gastric mucosa in the sample serves as an ideal internal control for staining intensity.
4. Result Reporting:
- Specify the sample type and location.
- Indicate whether the sample is adequate.
- Report the antibody clone and staining reagent used.
- Document the percentage of tumor cells with moderate-to-strong membrane staining, with a positivity threshold of 75%.