Primary Antibody Functions Explained: Key Differences from Secondary Antibodies

By admin
What Is a Primary Antibody: Definition and Core Function in Immunodetection
A primary antibody is an immunoglobulin that has a specific affinity to a target antigen—most typically a protein—of a biological specimen. It is the initial detection layer of immunoassays like immunohistochemistry (IHC), Western blotting, and ELISA. The primary function of the primary antibody is to recognize and bind to the target epitope of the antigen.
Celnovte is committed to the production of IVD-grade immunohistochemistry primary antibodies. The antibodies are produced on murine monoclonal or human monoclonal platforms, which ensure specificity and reproducible performance.
How Primary Antibodies Bind to Target Antigens
Primary antibodies attach to antigens through their antigen-binding sites, which are complementarily oriented to fit on an epitope of the target molecule. This high-affinity binding is the foundation for further detection, either directly using labeled primary antibodies or indirectly with secondary antibodies.
What Is a Secondary Antibody
Purpose of Signal Amplification
Secondary antibodies are produced to bind specifically to primary antibodies. They bear such labels as fluorophores or enzymes (e.g., HRP) that can be detected. The signal amplification is their main role—binding many secondary antibodies to a single primary antibody increases the signal intensity immensely.
How Secondary Antibodies Work on Primary Antibodies
Secondary antibodies recognize the Fc region of primary antibodies and are species-specific, e.g., anti-mouse secondary antibodies will only cross-link mouse-originated primary antibodies. Yes, usually they are grouped. This renders experimental design flexible and compatible with most detection systems.
Primary and Secondary Antibody Important Differences
Binding Specificity and Target Recognition
Primary antibodies bind directly to the target antigen with high specificity, while secondary antibodies bind indirectly by binding to the constant region (Fc) of the primary antibody.
Direct vs Indirect Detection
Direct detection uses labeled primary antibodies for simpler protocols but decreased sensitivity. Indirect detection uses unlabeled primary and labeled secondary antibodies, with increased signal amplification using many secondary attachments per primary antibody.
Signal Strength and Visualization
Indirect techniques yield stronger signals as various secondary antibodies can bind to one primary antibody, thus making it more visible. This is especially important for low-abundance targets where signal strength is important.
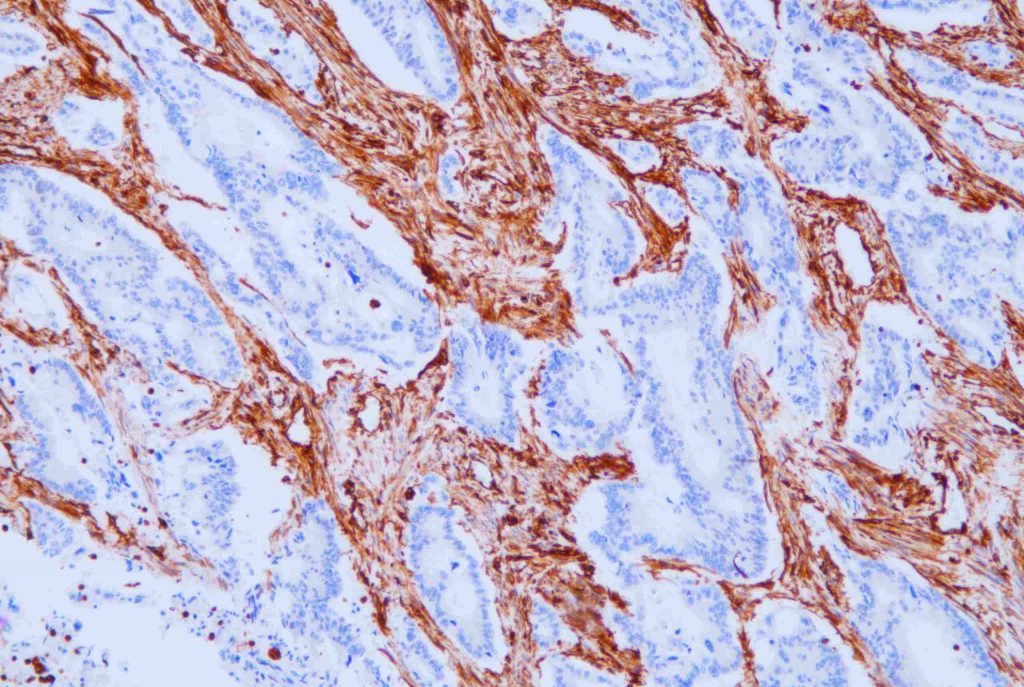
Practical Applications of Primary Antibodies
Use in Immunohistochemistry (IHC)
In IHC, the primary antibodies recognize specific proteins in tissue sections. Celnovte Biotech’s complete immunohistochemistry solution now consists of more than 460 primary antibodies. These reagents are compatible with paraffin-embedded as well as frozen tissue sections and allow for proper localization of biomarkers such as HER2 or Ki-67.
Role in Western Blotting and ELISA
Primary antibodies in Western blotting identify target proteins separated by electrophoresis and are subsequently identified through labeled secondary antibodies. In ELISA tests, they capture or detect antigens adsorbed on plates and play a crucial role in the quantification process.
Choosing the Right Primary Antibody from Celnovte
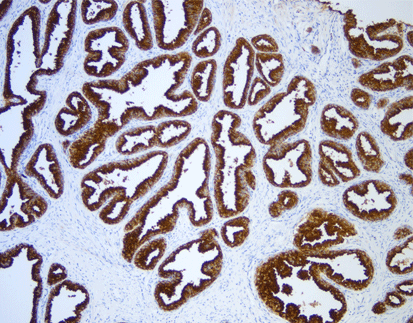
Product Overview: Celnovte HER2(C1F7)
The Celnovte HER2(C1F7) is a murine monoclonal antibody developed using hybridoma technology that targets human HER2 protein—a critical biomarker in breast cancer diagnosis and therapy guidance. Currently, it has developed nearly 80 antibodies, and ER obtained a Class I registration certificate approved by NMPA in 2022. This product has passed NordiQC quality control evaluation activities, ensuring reliability for clinical applications.
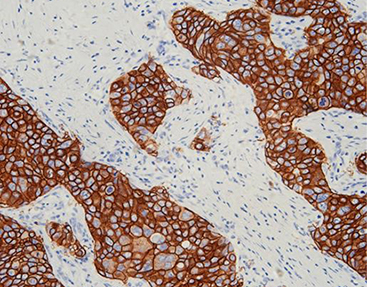
Prouct Overview: Celnovte Vimentin(V9)
Celnovte Vimentin(V9) is another high-performance monoclonal antibody used widely in pathology for identifying mesenchymal origin tumors or epithelial-mesenchymal transitions (EMT). It offers strong specificity with minimal background noise.
Advantages of Using Celnovte Primary Antibodies
High Specificity and Low Background Noise
The antibody engineering expression platform has the advantages of good repeatability, small batch-to-batch differences, and high yield. This ensures that each batch maintains consistent performance with low background staining—essential for diagnostic accuracy.
Compatibility with Multiple Detection Systems
Celnovte’s products are compatible with various detection systems, including enzyme-linked polymers and fluorescence-based multiplexing platforms like Akoya’s Opal system. Through careful protocol design, CNT’s mIHC kit consistently delivers stronger signal intensities.
Common Mistakes When Using Primary Antibodies
Incorrect Dilution or Incubation Time
Using incorrect dilutions can lead either to weak signals or non-specific binding, resulting in high background noise. Always refer to product datasheets for recommended concentrations and incubation durations.
Incompatible Blocking Buffers or Detection Systems
Blocking buffers must be compatible with both the tissue type and detection system used; otherwise, non-specific binding may occur. Our fixative has been verified by a large number of clinical practices. If replacement is needed, the department needs to verify it before clinical use.
Troubleshooting Guide for Poor Signal or High Background
Checklist for Reagent Compatibility and Protocol Optimization
Confirm species compatibility between your primary and secondary antibodies.
Ensure proper fixation techniques; avoid over-fixation that may mask epitopes.
Use validated blocking buffers.
Optimize washing steps; insufficient washes can retain unbound reagents.
Check expiration dates on all reagents, including DAB substrates.
Validate incubation times at room temperature or specified conditions.
If there is a discrepancy between intraoperative and postoperative diagnoses, a comprehensive judgment is made.
FAQ
Q: What happens if I use an incorrect dilution of my primary antibody?
A: Incorrect dilution can lead to either very faint staining (too diluted) or excessive background noise (too concentrated). Always follow manufacturer guidelines based on tissue type.
Q: Can I use any fixative with Celnovte’s primary antibodies?
A: Our fixative has been verified by a large number of clinical practices. If replacement is needed, the department needs to verify it before clinical use. Using unverified fixatives may compromise staining results.
Q: Are Celnovte’s HER2(C1F7) suitable for frozen section analysis?
A: HER2 guidelines explicitly require application in paraffin samples, so they cannot be used for treatment in frozen sections.
Q: Why do I need both primary and secondary antibodies?
A: While some assays allow direct labeling on primaries, using both enhances signal strength through amplification provided by multiple secondaries binding each primary.
RELATED PRODUCTS