How Ki-67 Marker Analysis Aids Early Detection of HPV Effects

By admin
The Ki-67 Role in HPV Detection Insight
What is the Ki-67
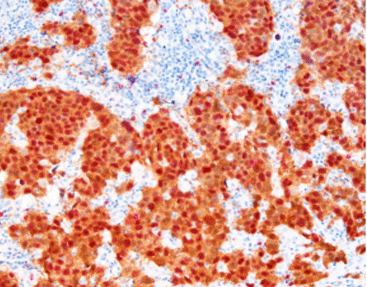
Ki-67 is a nuclear protein whose connection with cell division is quite strong. It shows up in every active part of the cell cycle, such as G1, S, G2, and mitosis. Yet, it stays missing in calm cells, like G0. Due to this feature, it works as an excellent indicator for dividing cells. In tissue samples, the appearance of Ki-67 lets people figure out how fast a tumor spreads or when strange cell actions occur.
Why Ki-67 is Useful in HPV-Associated Screening
In human papillomavirus (HPV) infection, particularly dangerous types linked to cervical cancer, Ki-67 acts as a valuable sign. Ki-67 points out the unusual expansion of lining cells. And it could serve as an initial clue for CIN or for heading toward malignancy. When joined with indicators like p16, Ki-67 proves even handier for telling apart safe areas from potentially harmful ones.
This method enables the identification of cervical precancerous lesions by highlighting both biomarkers on one slide from tissue or cell samples.
How Ki-67 Shows Abnormal Cell Growth
Association Between Ki-67 Expression and Cervical Lesions
Elevated Ki-67 levels are often found in cervical tissue exposed to high-risk HPV strains. Microscopic examination reveals that stronger nuclear staining for Ki-67 corresponds with lesion severity, from low-grade squamous intraepithelial lesions (LSIL) to high-grade (HSIL). Thus, Ki-67 is key for risk stratification and treatment planning.
Applications of Ki-67 in the Identification of High-Risk HPV Effects
Ki-67 over expression is even more informative when assessed alongside p16. While p16 indicates viral oncogene-driven processes, Ki-67 confirms cellular proliferation. This dual analysis helps identify HPV cases likely to progress to cancer or regress naturally.
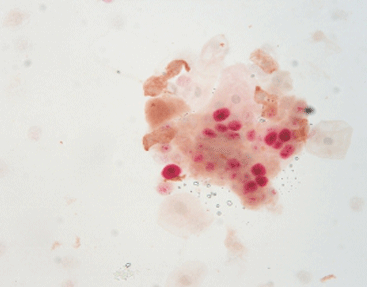
Especially useful is p16/Ki-67 cytological double staining. It depends on color-based results and is not influenced by subjective factors. This method is now widely applied in cervical cancer screening. It helps avoid missed or incorrect diagnoses caused by low sensitivity in cytology and limited HPV specificity. Ultimately, it improves the detection rate of the disease.
Advantages of Using Celnovte Ki-67 Detection Products
Overview of Celnovte Immunohistochemistry Reagents
Celnovte provides a complete immunohistochemistry solution. The company has mastered core IHC development technologies. It currently offers more than 460 primary antibodies. Additionally, it has independently created secondary antibody detection systems. The product range includes over 200 IHC quality control items. There are also fully automated staining platforms available. These tools help customers achieve high-quality staining outcomes. Such resources ensure reliable performance across clinical uses.
Features of Celnovte Ki-67 Dual Staining Detection Kit
The Celnovte p16/Ki-67 dual staining kit enables visualization of both markers on one slide through chromogenic techniques. This combined method lowers uncertainty in diagnosis and increases screening effectiveness.
p16/Ki-67 double staining detects two biomarkers, p16 and Ki-67, on a single slide using cell or tissue samples. This makes it perfect for standard cervical cancer screening processes. The kit offers a clear color contrast, which reduces subjectivity during result interpretation.
Compatibility with Celnovte Automated Staining Platforms
Our fully automated IHC staining machine can process up to 60 samples. It completes staining within 2.5 hours, offering high efficiency for busy pathology laboratories. The system also enables advanced applications such as multi-color IHC and chromogenic in situ hybridization.
This compatibility allows smooth incorporation into current lab setups. It maintains both high throughput and reproducible results.
Application in Early Diagnosis and Clinical Workflow
Integration into Routine Cervical Biopsy Analysis
Ki-67 marker-based dual staining kits from Celnovte can be easily added to standard pathological evaluations. These include cervical biopsies and cytology smears. The kits come with optimized protocols for both frozen and paraffin-embedded tissue samples. This provides flexibility across various sample types.
Since HE staining only offers morphological information, intraoperative immunohistochemistry can enhance diagnostic accuracy for pathologists.
Enhancing Diagnostic Accuracy with Celnovte Solutions
By integrating accurate antigen retrieval techniques with high-affinity antibodies produced through murine monoclonal hybridoma technology, Celnovte guarantees precise results. This is true even for challenging diagnostic cases.
The antibody engineering expression platform uses independently designed high-expression vectors. These provide benefits such as excellent repeatability and minimal variation between batches. Such consistency ensures stable outcomes across different test batches.
Supporting Pathologists in Decision-Making
Clear Visualization with High-Affinity Antibodies
Celnovte’s tailored antibody solutions offer outstanding specificity and sensitivity. This is due to their proprietary development platforms. The Celnovte Antibody R&D Center focuses on creating IVD-grade IHC primary antibodies. It uses murine monoclonal antibodies based on hybridoma cell fusion technology. These antibodies deliver a strong contrast during staining. This is crucial for accurate microscopy interpretation.
Consistent Staining Results Across Batches
Consistency between batches is essential for clinical trust. Celnovte’s quality control procedures follow GMP standards. We also hold international certifications like ISO13485 and CE-ID. We operate clean production workshops. It also has supporting R&D, manufacturing, and quality testing equipment. These resources ensure product uniformity at large scales. This is especially important when tests are used across multiple clinics or long-term studies.
FAQ
Q: What does positive Ki-67 expression indicate in HPV testing?
A: Positive expression points to active cell division. This is often seen in HPV-related dysplasia or early cancer development.
Q: How does dual p16/Ki-67 staining improve diagnostic accuracy over traditional methods?
A: It merges signs of viral oncogene activity (p16) with cell proliferation (Ki-67). This helps pathologists tell apart temporary infections from those that may become cancerous.
Q: Can Celnovte’s Ki-67 kits be used on both frozen sections and paraffin samples?
A: Yes. Although frozen IHC requires specific certifications—such as for HER2 and ER, which are officially recommended for paraffin samples—the raw materials remain the same. This ensures dependable results for both sample types.
RELATED PRODUCTS