The Role of ER and PR in Breast Cancer: Prognosis and Treatment Guide

By admin
For each patient facing a breast cancer diagnosis, the journey ahead involves many questions. Some of the most important ones are: “What kind of cancer is this?” and “What treatment suits me best?” The responses often come from the tumor cells. They focus on the presence or lack of two main biomarkers: the Estrogen Receptor (ER) and the Progesterone Receptor (PR). Precise evaluation of these receptors is more than a diagnostic phase. It forms the basis of customized breast cancer treatment. This overview examines the essential function of ER and PR. It also presents the sophisticated instruments that guarantee every diagnosis reaches the utmost level of assurance.
Understanding Hormone Receptors in Breast Cancer
What Are ER and PR?
Estrogen and progesterone are natural hormones in the body. In certain breast cancers, the cancer cells possess receptors—proteins—that connect to these hormones. The cells then use them as energy to expand and divide.
• Estrogen Receptor (ER)-positive breast cancer indicates that the cancer cells develop due to estrogen.
• Progesterone Receptor (PR)-positive breast cancer shows that the cancer cells develop due to progesterone.
A cancer might be positive for one receptor, both, or none. This grouping, called the hormone receptor (HR) status, is basic to grasping the tumor’s conduct.
Why Testing is a Critical First Step
Figuring out the HR status of a breast tumor matters for two primary reasons.
1. Prognosis: In general, HR-positive breast cancers grow at a slower pace. They also tend to have a stronger outlook than HR-negative ones.
2. Treatment: This stands as the key element. HR-positive cancers often respond well to hormone therapy (also known as endocrine therapy). Such treatment aims to hinder hormone effects or reduce their amounts in the body.
The Gold Standard: Immunohistochemistry (IHC) for ER/PR Detection
The IHC Workflow: From Tissue to Diagnosis
To assess the ER/PR status, pathologists apply a strong method named Immunohistochemistry (IHC). This approach shows the presence and position of the ER/PR proteins in a tissue specimen. The process includes making thin sections of the tumor tissue. Next, experts add specific primary antibodies that attach only to the ER or PR proteins. Then, they employ a detection setup to produce a noticeable stain at the binding sites. After that, a pathologist reviews the stained slide with a microscope to decide the outcome.
The Challenge: The Need for Uncompromising Accuracy
The full care plan for a patient depends on the correctness of this IHC test. A wrong negative result might prevent a patient from getting a vital hormone therapy. Meanwhile, a wrong positive could result in useless treatment. As a result, each part of the IHC process—from the antibodies to the staining devices—must work perfectly.
Celnovte’s Integrated Solution for Precision ER/PR Testing
At Celnovte, we know that exactness cannot be compromised. We offer a broad collection of top IHC supplies and devices. These are built to operate together without issues. They provide sharp, steady, and dependable outcomes for ER and PR checks.
Superior Primary Antibodies: The Foundation of Accuracy
The primary antibody serves as the most vital supply in the IHC method. Its capacity to attach precisely and firmly to its target protein sets the test’s correctness. Celnovte’s self-cloned ER and PR primary antibodies excel due to their outstanding quality and function. They are not merely dependable. Their superiority receives outside confirmation.
• Proven Excellence: Our primary antibodies have gained an “optimal” score in NordiQC reviews for six straight years. NordiQC (Nordic Immunohistochemical Quality Control) acts as a worldwide expert on IHC quality checks. This ongoing high-level result shows unmatched focus and dependability.
• Certified for Clinical Use: Our ER and PR antibodies have also obtained Class III NMPA approval. This ensures they satisfy the top regulatory rules for in-vitro diagnostic purposes.
When you select Celnovte antibodies, you choose supplies shown to give the correct response, over and over.
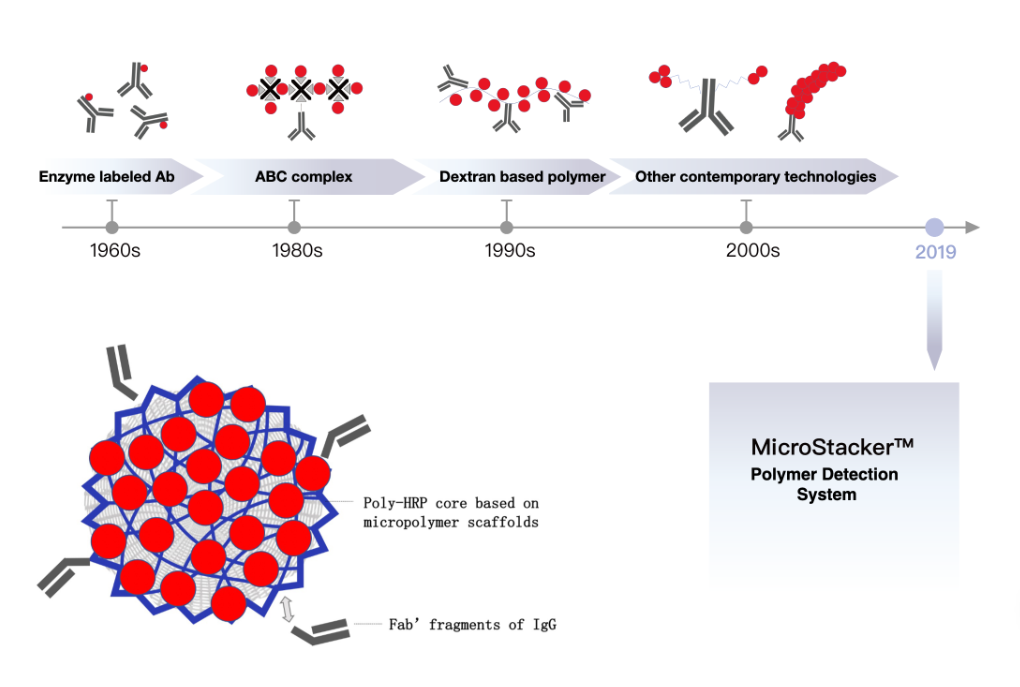
Enhanced Sensitivity with MicroStacker™ Detection Systems
Spotting the antibody-protein link needs a strong and clean detection setup. Celnovte’s own MicroStacker™ Plus Polymer Detection Kits are designed for top function. This sophisticated method offers a strong signal with little background color. It leads to clear, definite views. Such sharpness is vital for pathologists. They use it to rate the portion and strength of stained cells accurately. In turn, this brings a more assured diagnosis.
Automated Consistency with High-Performance IHC Stainers
Manual coloring can bring in differences and requires much effort for busy labs. Celnovte’s lineup of automated IHC Stainers handles these issues. They do so by offering standard, hands-free handling.
• Reproducibility: Our devices make sure each slide follows the same exact steps. This removes mistakes from people and secures steady results across runs.
• Efficiency: Automation saves precious time for technicians. It also raises the output of your lab. As a result, you can send important findings to doctors and patients sooner.
From our top-function antibodies and MicroStacker™ detection setups to our dependable IHC stainers, Celnovte supplies a full and tuned solution for ER/PR checks.
Interpreting the Results: Guiding Patient Care
Prognostic Significance of ER/PR Status
When a pathology summary returns, it will label the tumor as ER-positive/negative and PR-positive/negative. This detail aids in forecasting the probable result and actions of the cancer.
Tailoring Treatment with Hormone Therapy
The HR status straightaway shapes the best care approach.
• For HR-positive (ER+ and/or PR+) breast cancer, hormone therapy acts as a main treatment choice. These medicines, like tamoxifen or aromatase inhibitors, work well at stopping cancer from returning.
• For HR-negative breast cancer, hormone therapies do not help. These patients move toward other options, such as chemotherapy or targeted drugs.
Celnovte: Your Partner in Pathological Diagnostics
Started in 2010, Celnovte Biotechnology leads worldwide in creating and producing diagnostic supplies and devices. With research and production sites in China and the USA, our work meets the highest global quality rules. These include NMPA, GMP, ISO13485, ISO9001, and CE approvals. Our goal is to equip labs with the resources they require for assured and precise diagnostics.
Conclusion
The functions of ER and PR in breast cancer are clear. They direct both outlook and vital treatment choices. Gaining a correct diagnosis relies fully on the standard of the tools in the pathology lab. Celnovte supplies a completely linked system of elite primary antibodies, sophisticated detection setups, and automated devices. By picking Celnovte, you select a collaborator committed to exactness, dependability, and, in the end, improved patient results.
To find out more about our broad IHC options for breast cancer diagnostics, reach out to us or check our product lineup today.
FAQ
Q: Why is accurate ER/PR testing so important?
A: Accurate ER/PR testing matters greatly because it determines if a patient gains from hormone therapy. This stands as one of the strongest treatments for HR-positive breast cancer. A wrong outcome can cause less-than-ideal or useless care.
Q: What makes Celnovte’s ER/PR antibodies reliable?
A: Our ER and PR antibodies have steadily earned “optimal” marks in NordiQC outside quality checks for six straight years. This separate proof, plus Class III NMPA approval, verifies their strong focus, awareness, and dependability in medical environments.
Q: What are the benefits of using an automated IHC stainer from Celnovte?
A: Celnovte’s automated IHC stainers bring major advantages. These include better steadiness and repeatability by removing hand differences. They also improve lab productivity and output. Plus, they cut manual time for technicians.
RELATED PRODUCTS