How to Optimize Your Multiplex IHC Workflow for Consistent Results
 2025-10-09
2025-10-09
By admin

In the quickly changing field of molecular pathology, the skill to gather full details from small tissue samples is vital. Multiplex Immunohistochemistry (IHC) leads this progress, letting experts and doctors view several biomarkers at once in one tissue piece. This strong method gives unmatched views into cell links, illness growth, and treatment reactions. It goes well beyond the limits of regular single-plex IHC. But reaching the complete strength of Multiplex IHC depends on improving each part of the process. This ensures steady, trustworthy, and repeatable outcomes.
The Power and Promise of Multiplex IHC
Unlocking Deeper Biological Insights
Multiplex IHC offers a detailed picture of data. It allows the simultaneous spotting of many antigens on one slide. This skill is key to grasping complex life systems. It helps find rare cell groups and understand detailed signaling paths. For diagnosis, it makes the review of many biomarkers easier. It saves valuable tissue, too. Plus, it lets users check space links between various cell kinds. Such checks often prove critical for the right diagnosis and outlook.
Navigating the Challenges of Consistency
Even with its deep benefits, getting steady and top-quality outcomes in Multiplex IHC can prove tough. The detailed task of spotting many targets brings issues. These include handling possible antibody mix-ups, keeping good signal-to-noise levels, and holding batch-to-batch sameness. All this needs close care at each stage. Uneven outcomes can cause wrong understandings. Such errors might slow key study findings or harm patient health. So, a strong and improved process is not just helpful. It stands as necessary.
Core Pillars of an Optimized Multiplex IHC Workflow
Improving your Multiplex IHC process calls for a planned method. It focuses on several main areas. These range from sample reading to end data review.
1. Meticulous Sample Preparation and Antigen Retrieval
The base of any winning IHC test is well-prepared tissue. This covers careful tissue gathering, fixing (e.g., formalin-fixed paraffin-embedded, FFPE), and cutting. Antigen retrieval plays a big role in multiplexing. It uncovers epitopes hidden by fixing. Improved retrieval steps make sure all target antigens stay open to their antibodies. Yet, they do not harm tissue shape or other epitopes. Changes in these first steps can greatly affect the steadiness of later outcomes.
2. Leveraging High-Performance Reagents and Detection Systems
The grade and exactness of primary antibodies and detection setups are firm for steady Multiplex IHC. Using checked primary antibodies with shown exactness for their targets matters a lot. This holds even in a multiplex setting. It helps dodge false positives or negatives. Just as key are sensitive and strong detection setups. They boost signals well while cutting background sounds.
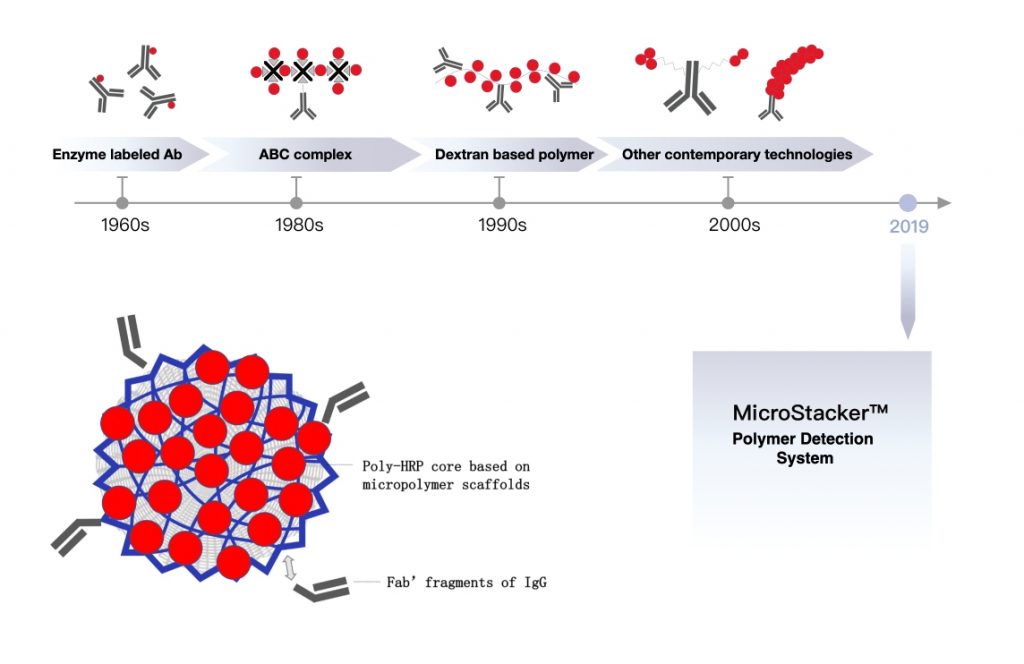
3. Introducing Celnovte’s MicroStacker™ Plus Polymer Detection Kit
At Celnovte, we grasp the main role of better detection. Our MicroStacker™ Plus Polymer Detection Kit leads the market. It comes backed by owned smart property. We built it to give outstanding sensitivity and exactness in IHC uses. This forward polymer-based setup ensures strong, clear signals with little background. Such traits make it perfect for the tough needs of Multiplex IHC. There, a clear split of many markers proves vital. Its top work adds greatly to the steadiness and trustworthiness of your outcomes. It does this by giving a sturdy and repeatable signal. Celnovte also holds a market-leading group of IHC reagents. These include ones with MicroStacker™ technology.
4. Achieving Precision with Automated Staining Platforms
Manual IHC staining sometimes proves needed. But it brings changes due to human factors. These include uneven reagent use, timing, and heat. For Multiplex IHC, where exact control over each step grows bigger, automation turns into a strong tool for steadiness.
5. The Role of Celnovte IHC Stainers
Celnovte’s lineup of IHC Stainers gives automated fixes. We made them to set standards and smooth your staining process. Our tools ensure exact reagent giving, steady incubation times, and improved heat control across all slides. This greatly cuts down changes and human slips. Whether you run a high-output lab or a smaller spot, Celnovte provides various models. These include small ones that meet CLIA rules. They fit diverse needs. By automating the staining process, our IHC Stainers help reach unmatched sameness and speed. They free up key worker time, too. Plus, they ensure steady outcomes from test to test and patient to patient. This proves extra vital in high-output labs. There, manual vs. automated stainers greatly affect time, cost, and slip rates.
6. Advanced Digital Pathology for Imaging and Analysis
The last stage of the process involves imaging and review. Multiplex IHC creates complex data. It often needs forward digital pathology fixes. High-detail digital scanners that catch multi-spectral pictures prove necessary. They help to separate many fluorophores or chromogens. Advanced image review software then measures biomarker signals, checks space links, and gives objective, steady data. This moves past personal visual takes.
Celnovte’s Integrated Solutions for Multiplex IHC
Celnovte Biotechnology stays committed to giving full fixes. These span the whole pathology process, from reagents to tools and digital pathology. Our joint method ensures a match and smooth work. It handles the special challenges of Multiplex IHC.
Targeted Diagnostics with Celnovte’s TTF-1/Napsin A and CK5/6/p40 Reagents
Look at our TTF-1/Napsin A detection reagent (Immunohistochemical) and CK5/6/p40 detection reagent (Immunohistochemical). These serve as top examples of how our special reagents boost the exactness. The TTF-1/Napsin A panel proves key for splitting lung adenocarcinomas. There, both markers show up. It sets them apart from other lung tumors. In the same way, the CK5/6/p40 panel proves valuable in telling squamous cell carcinomas (CK5/6 and p40 positive) from adenocarcinomas (negative for both) in various tumor kinds. By giving pre-checked, improved dual-staining reagents, Celnovte helps make simple, complex split diagnoses. It ensures the right phenotyping and cuts turnaround time. All this happens while keeping the high steadiness demanded by clinical and study settings. Other examples include CK5/6/p63 and Napsin A/p40 detection reagents.
Our Commitment to Comprehensive Pathology Solutions
Our product group spreads across Immunohistochemistry, Instruments, Histology, Molecular, Cytology, and Digital Pathology. It offers a whole method for lab needs. This full offering makes sure every part of your process gets backed by top-grade, trustworthy fixes. We also give Clinical Solutions and Research Solutions.
Partnering for Pathological Advancement
Why Choose Celnovte Biotechnology?
As a leader in molecular and tumor pathology diagnostics, Celnovte has focused on new ideas since our start in 2010. Based in Zhengzhou, China, with R&D spots in Shenzhen, Suzhou, and Maryland (USA), we hold a worldwide reach and a pledge to science top work. We are making spots in China to meet NMPA & GMP rules. Plus, we have ISO13485 and ISO9001 marks. These ensure the highest levels of grade and trustworthiness for all our items. Contact us today to learn more about our full fixes.
FAQ
Q: What is the main advantage of Multiplex IHC over traditional IHC?
A: Multiplex IHC allows for the simultaneous detection of multiple biomarkers on a single tissue section, conserving tissue and providing insights into spatial relationships between different cellular components that traditional single-plex IHC cannot offer.
Q: How do Celnovte’s IHC Stainers contribute to workflow optimization?
A: Our IHC Stainers automate the staining process, ensuring consistent reagent application, incubation times, and temperature control, which significantly reduces human error and enhances reproducibility and efficiency across all experiments.
Q: Are Celnovte’s products suitable for both research and clinical applications?
A: Yes, Celnovte provides both Clinical Solutions and Research Solutions. Our products are designed to meet stringent quality standards, including NMPA & GMP compliance, and ISO13485 and ISO9001 certifications, making them suitable for various professional settings.
RELATED PRODUCTS






