PRODUCT CATEGORY
PRODUCT CATEGORY
CONTACT US
Phone
Email
Address
6 Cuizhu St, Zhong Yuan Qu, Zheng Zhou Shi, He Nan Sheng, China, 450001
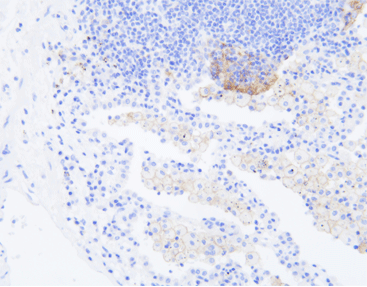
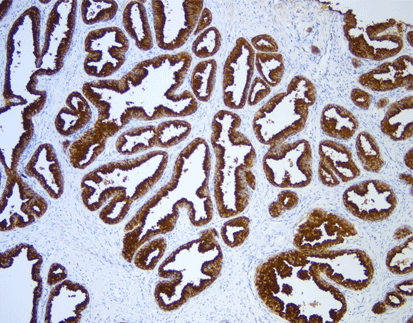
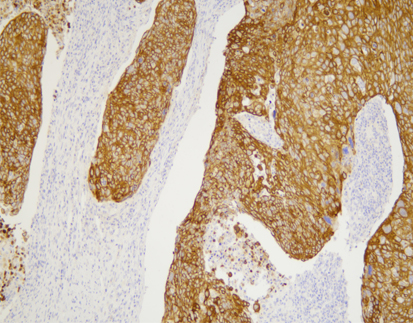
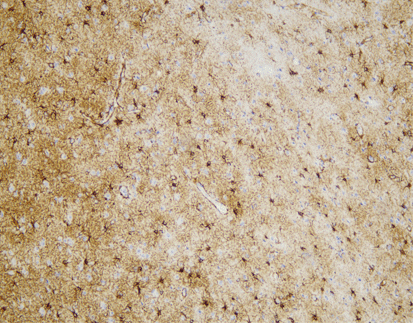
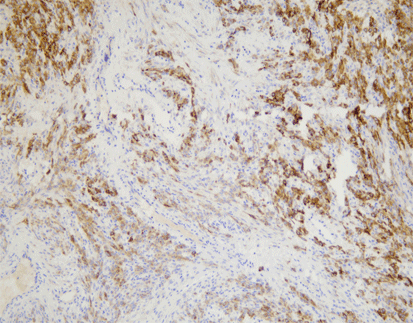
PD-L1 (C9C9), MMab
Discover the crucial role of the PD-L1 protein in cancer immunity. PD-L1 helps tumors evade immune responses by binding to the PD-1 receptor on T-cells, making them resistant to CD8+ T cell-mediated lysis. Assessing PD-L1 expression, especially alongside CD8+ tumor-infiltrating lymphocytes (TILs), is vital in cancers like stage III NSCLC, hormone receptor-negative breast cancer, and sentinel lymph node melanoma.
For In vitro diagnostics use.
Research Application
[As Shown in Picture]
We’re proud to announce that our self-developed primary PD-L1 (C9C9) antibody has won an optimal rating in the prestigious NordiQC assessment. This recognition underscores our commitment to advancing cancer diagnostics with precision and reliability.
Key usages of PD-L1 marker in IHC:
– Cancer Diagnosis: Identifies PD-L1 expression in tumor tissues.
– Treatment Decisions: Guides the use of immunotherapies, particularly PD-1/PD-L1 inhibitors.
– Prognostic Value: Provides insights into the likely outcomes of immunotherapy.
– Precision Medicine: Tailors treatments to individual patient profiles, enhancing the efficacy of targeted therapies.
[Intended Use]
Discover the crucial role of the PD-L1 protein in cancer immunity. PD-L1 helps tumors evade immune responses by binding to the PD-1 receptor on T-cells, making them resistant to CD8+ T cell-mediated lysis. Assessing PD-L1 expression, especially alongside CD8+ tumor-infiltrating lymphocytes (TILs), is vital in cancers like stage III NSCLC, hormone receptor-negative breast cancer, and sentinel lymph node melanoma.
[Specifications]
| Product Name | PD-L1 (C9C9), MMab |
| Catalog No. |
|
| Intended Use | IVD, RUO |
| Species Reactivity | Human; others not tested |
| Cellular Localization | Cytoplasm / Nucleus |
| Antibody Type | Mouse Monoclonal |
| Clone | C9C9 |
| Format and Volume | Ready-to-use: 1mL, 3mL, 6mL Concentrated: 0.1mL, 0.2mL and 1mL |
[Datasheets & SDS]
| IVD Datasheet (IFU) | ↕️ Download |
| RUO Datasheet (IFU) | ↕️ Download |
| SDS sheet | check with sales |
[Storage and Validity]
Store at 2~8°C. Avoid freezing.
Maintain temperature below room temperature during transport, ensuring it does not exceed one week.
References:
1. Ostrand-Rosenberg S, Horn LA, Haile ST. The programmed death-1 immunesuppressive pathway: barrier to antitumor immunity. J Immunol. 2014 Oct 15;193
(8):3835-41.
2. Tokito T, et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent
chemoradiotherapy. Eur J Cancer. 2016 Jan 6;55:7-14.
3. Park IH, et al. Prognostic implications of tumor-infiltrating lymphocytes in association with programmed death ligand 1 expression in early-stage breast cancer. Clin Breast Cancer. 2016 Feb;16(1):51-8.
4. Kakavand H, et al. Tumor PD-L1 expression, immune cell correlates and PD-1+ lymphocytes in sentinel lymph node melanoma metastases. Mod Pathol. 2015 Dec;28
(12):1535-44.
5. Xia B, Herbst RS. Immune checkpoint therapy for non-small-cell lung cancer: an update. Immunotherapy. 2016 Feb 9 [Epub ahead of print].
6. Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015 Apr;14(4):847-56.
7. Singh BP, Salama AK. Updates in therapy for advanced melanoma. Cancers (Basel). 2016 Jan 15;8(1).
8. Center for Disease Control Manual. Guide: Safety Management, NO. CDC-22, Atlanta, GA. April 30, 1976 “Decontamination of Laboratory Sink Drains to Remove Azide Salts.”
9. Clinical and Laboratory Standards Institute (CLSI). Protection of Laboratory Workers from Occupationally Acquired Infections; Approved Guideline-Fourth Edition CLSI document M29-A4 Wayne, PA 2014.